Session Information
Date: Sunday, October 26, 2025
Title: (0337–0356) Osteoporosis & Metabolic Bone Disease – Basic & Clinical Science Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Patients with Rheumatoid Arthritis (RA) have an increased risk of osteoporosis and fragility fractures. Bone loss in RA is multifactorial and mainly determined by classical risk factors for fracture (age, previous fracture, parental hip fracture, postmenopausal period duration, proximal femur bone mineral density (BMD)), factors associated with RA (disease activity, erosions, disability) and use of glucocorticoids (GC). A recent study (Bone. 2023;168:116654) suggested that patients with RA treated with Rituximab (RTX) may have a higher incidence of clinical fragility fractures (CFF), compared to those receiving other DMARDs. However, this association has not been thoroughly studied.
Methods: Retrospective, single-centre observational study in patients with RA diagnosis, according to the ACR/EULAR 2010 RA classification criteria, who received at least one dose of RTX between 1 January 2010 and 31 December 2019. Two comparator groups matched by sex, age, and disease duration were included: one treated with conventional synthetic DMARDs (group 1), and the other with biological or targeted synthetic DMARDs (group 2). Patients treated with RTX were assigned to group 3. Hospital and primary care medical records were reviewed up to 31 December 2024 or until loss of follow-up. We defined CFF as those resulting in the absence of external injury, such as from falls from one’s own height or walking at normal speed. Date and location of CFF were recorded, as well as the date and reason for the end of follow-up, together with data on RA characteristics and fracture risk factors.
Results: A total of 204 patients were included (68 per group), of whom 71% were women, with a mean age of 53.6 years and a mean disease duration of 2.0 years, with no significant differences between groups. The indication for RTX was RA disease activity in 62% of cases and interstitial lung disease (ILD) in 38%. Baseline characteristics of the groups are summarised in Table 1.Fifty-eight patients had 90 CFF (39 vertebral fractures, 13 humerus, 10 distal forearm, 4 hip fractures, and 24 other locations). The incidence of CFF was 2.1, 2.4 and 2.3 per 100 person-years (ppy), in groups 1, 2 and 3 respectively, with no statistically significant differences. Patients with CFF showed a significantly higher prevalence of erosions, parental history of hip fracture, use of GC, WHO osteoporosis diagnostic category, lower BMD at lumbar spine, femoral neck and total hip, and higher FRAX scores.The mortality rate was 0.7, 0.7 and 2.8 ppy (p< 0.001), respectively. In group 3, mortality was particularly high when RTX was indicated for ILD (53.9%) rather than for RA disease activity (11.9%) and in patients with vertebral fractures.
Conclusion: In our sample, there were no differences in the incidence of CFF between groups treated with RTX or other DMARDs. CFF were associated with classical fracture risk factors and erosive disease. The group treated with RTX showed higher mortality, particularly when the indication was ILD and in patients with vertebral fractures.
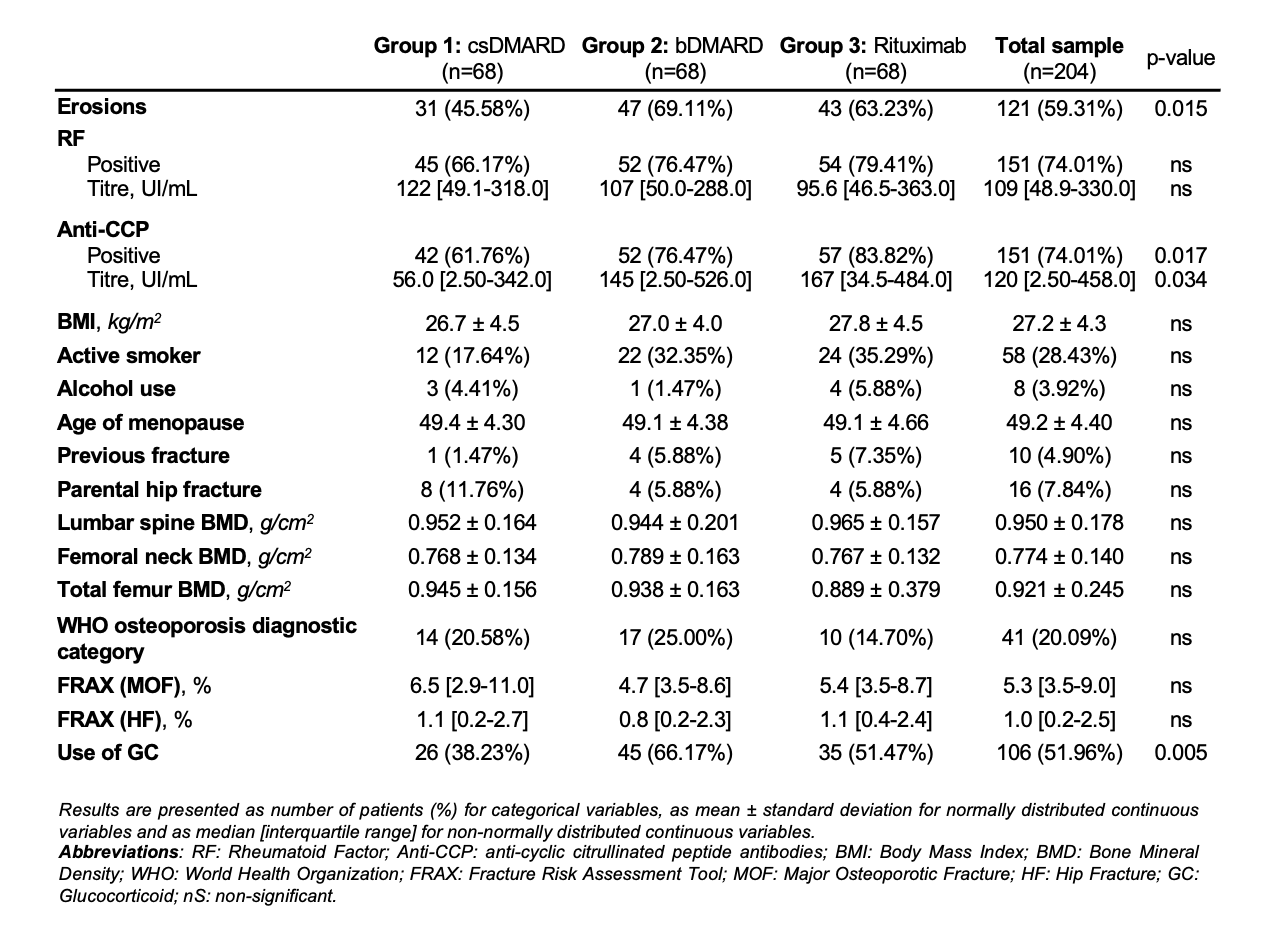 Table 1: RA characteristics and fracture risk factors, at baseline.
Table 1: RA characteristics and fracture risk factors, at baseline.
To cite this abstract in AMA style:
Roig Kim M, Marco-Pascual C, Aguilar-Coll M, Narváez J, Nolla J, Gomez Vaquero C. Incidence of Clinical Fragility Fractures and Mortality in Patients with Rheumatoid Arthritis Treated with Rituximab [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/incidence-of-clinical-fragility-fractures-and-mortality-in-patients-with-rheumatoid-arthritis-treated-with-rituximab/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/incidence-of-clinical-fragility-fractures-and-mortality-in-patients-with-rheumatoid-arthritis-treated-with-rituximab/
