Session Information
Date: Sunday, October 26, 2025
Title: (0337–0356) Osteoporosis & Metabolic Bone Disease – Basic & Clinical Science Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Osteoporosis is the most common bone metabolism disorder in developed countries. It increases the risk of fragility fractures, impairing quality of life, increasing disability, mortality, and healthcare costs. Recently approved, Romosozumab is the only treatment with dual antiresorptive and bone-forming effects.The aim of this study is to characterize the profile of patients treated with romosozumab, as well as its safety and efficacy.
Methods: This is a retrospective, longitudinal, descriptive study of patients with osteoporosis treated with romosozumab. Baseline clinical data on bone metabolism (PTH, β-CTX, P1NP, ALP, and vitamin D) and densitometry (T-score/BMD) were collected at the start of treatment, at 6 months, and after completing 1 year.
Results: A total of 49 patients (100% women) with a mean age of 71.8 years were included. Primary osteoporosis was diagnosed in 75.5% of cases, and 70.2% had a history of fractures, most commonly vertebral (57.6%). The most frequent cardiovascular risk factors were dyslipidemia (42.9%), hypertension (40.8%), and diabetes (8.2%). Active smoking was reported by 8.2% of patients, and none reported habitual alcohol consumption. A history of malignancy was recorded in 10 patients (3 non-melanoma skin cancer, 3 breast, 1 lung, 1 kidney, 1 hematologic, and 1 parotid), and 5 patients had a history of radiotherapy.Prior to romosozumab, the most commonly used treatments were oral bisphosphonates (38.8%), denosumab (20.4%), and teriparatide (16.3%). The mean duration of romosozumab treatment was 10.51 months (median: 12 months). A total of 5 adverse events were recorded: 1 case each of arthromyalgia, heart failure decompensation, dizziness, blurred vision, and local reaction. No pulmonary embolism (PE), major adverse cardiovascular events (MACE), or strokes were reported. Of the 49 patients who initiated treatment, 28 completed the full 12-month course. The most frequently used medications after romosozumab were denosumab (44.4%), risedronic acid (27.8%), zoledronic acid (13.9%), alendronate (5.6%), and teriparatide (2.8%).Renal function markers and general biochemical markers remained stable throughout treatment. Regarding bone metabolism markers, β-CTX decreased from 0.33 to 0.23, as did ALP (100.12 to 90.85). Conversely, P1NP increased (from a mean of 47.94 to 59.18). PTH increased at 6 months but returned to normal by the end of treatment. Vitamin D levels remained unchanged.For densitometry, the mean baseline T-scores were: lumbar spine -3.05, femoral neck -2.63, and total hip -2.15. Among patients who completed 12 months of treatment, BMD increased at all three sites. This resulted in a reduction in the proportion of patients with osteoporosis: from 70.45% to 46.67% at the lumbar spine, from 60% to 50% at the femoral neck, and from 34.88% to 21.43% at the total hip.
Conclusion: Romosozumab is a safe bone-forming treatment option for elderly patients with established classic cardiovascular risk factors. An increase in bone formation markers and a reduction in bone resorption were observed in blood tests, along with an increase in BMD. The most frequently used therapy after romosozumab was denosumab, followed by bisphosphonates.
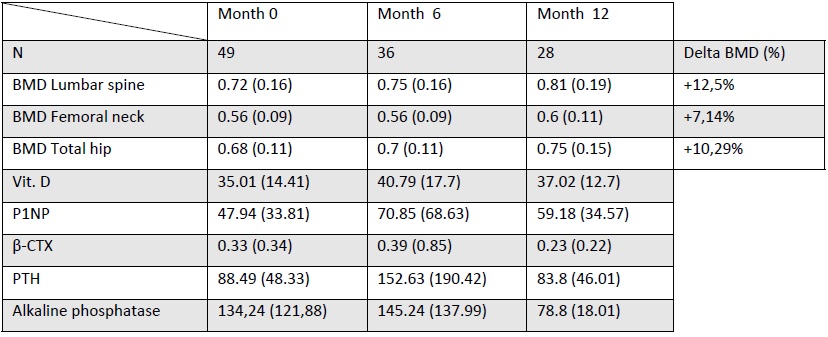 Table 1. BMD and bone biomarkers evolution during Romosozumab treatment.
Table 1. BMD and bone biomarkers evolution during Romosozumab treatment.
To cite this abstract in AMA style:
Ramos Castro D, LEAL S, GRAU GARCIA E, Muñoz-Martínez P, Mas Sanchez L, Torrat Noves A, Villanueva Manes B, Alcántara Álvarez I, perez Hurtado A, Simeo Vinaixa M, Andrés Román Ivorra J. Romosozumab in patients with Osteoporosis: Safety and Efficacy analysis in Clinical Practice [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/romosozumab-in-patients-with-osteoporosis-safety-and-efficacy-analysis-in-clinical-practice/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/romosozumab-in-patients-with-osteoporosis-safety-and-efficacy-analysis-in-clinical-practice/
