Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Methotrexate (MTX), traditionally used for inflammatory conditions like rheumatoid arthritis, has well-established anti-inflammatory properties, recently gained interest as a potential therapeutic option for osteoarthritis (OA). Current treatments largely focus on symptom management, with limited options for altering disease course. This study aims to evaluate the safety and efficacy of MTX in reducing OA symptoms in both the short and long term.
Methods: We systematically searched PubMed, Web of Science, Embase, Ovid, and Scopus from inception to April 22, 2025. Studies evaluating the safety and efficacy of MTX in OA were included (Fig. 1). The primary outcome was pain, measured by the Visual Analogue Scale (VAS) or Numeric Rating Scale (NRS). Outcomes were stratified by follow-up duration: short-term (less than 6 months), medium-term (6 months and 9 months), and long-term (12 months). Data were extracted and analyzed using Review Manager 5.4 (RevMan) software.
Results: This systematic review and meta-analysis included 14 studies in the qualitative synthesis and 8 in the meta-analysis, encompassing 1251 and 649 patients, respectively. MTX significantly reduced pain (SMD: –0.47; 95% CI: –0.68 to –0.26). Subgroup analyses by treatment duration showed SMDs of –0.46 (95% CI: –0.75 to –0.17) at < 6 months, –0.75 (95% CI: –1.2 to –0.29) at 6 months, –0.42 (95% CI: –1.35 to 0.51) at 9 months, and –0.06 (95% CI: –0.46 to 0.34) at 12 months. For the WOMAC score, MTX had a significant overall effect (SMD: –0.71; 95% CI: –1.2 to –0.22), with subgroup SMDs of –0.45 (95% CI: –0.88 to –0.01) at < 6 months, –1.81 (95% CI: –4.56 to 0.94) at 6 months, –0.37 (95% CI: –0.78 to 0.04) at 9 months, and –0.27 (95% CI: –0.68 to 0.13) at 12 months. For physical function, the overall effect was significant (SMD: –0.53; 95% CI: –0.84 to –0.23), with subgroup SMDs of –0.46 (95% CI: –0.76 to –0.16) at < 6 months, –0.88 (95% CI: –1.73 to –0.03) at 6 months, –0.35 (95% CI: –0.81 to 0.12) at 9 months, and –0.19 (95% CI: –0.58 to 0.19) at 12 months. On stiffness, MTX also had an overall significant effect (SMD: –0.44; 95% CI: –0.65 to –0.23). Subgroup SMDs were –0.33 (95% CI: –0.68 to 0.01) at < 6 months, –0.52 (95% CI: –0.81 to –0.24) at 6 months, –0.39 (95% CI: –1.49 to 0.72) at 9 months, and –0.34 (95% CI: –1.43 to 0.75) at 12 months (Fig. 2). Our results stated that there is an overall significant difference in adverse events (AEs) (RR, 0.75; 95% CI, 0.60 to 0.94), with a significant difference in mild AEs (RR, 0.77; 95% CI, 0.61 to 0.97) and no significant difference in serious AEs (RR, 0.65; 95% CI, 0.31 to 1.34) (Fig. 3).
Conclusion: MTX provides significant short- and medium-term improvements in pain, stiffness, and physical function in OA, with the most pronounced benefit observed at 6 months. However, its safety profile in real-world settings requires more thorough investigation. Further large-scale randomized controlled trials are needed to confirm these findings and assess long-term efficacy.
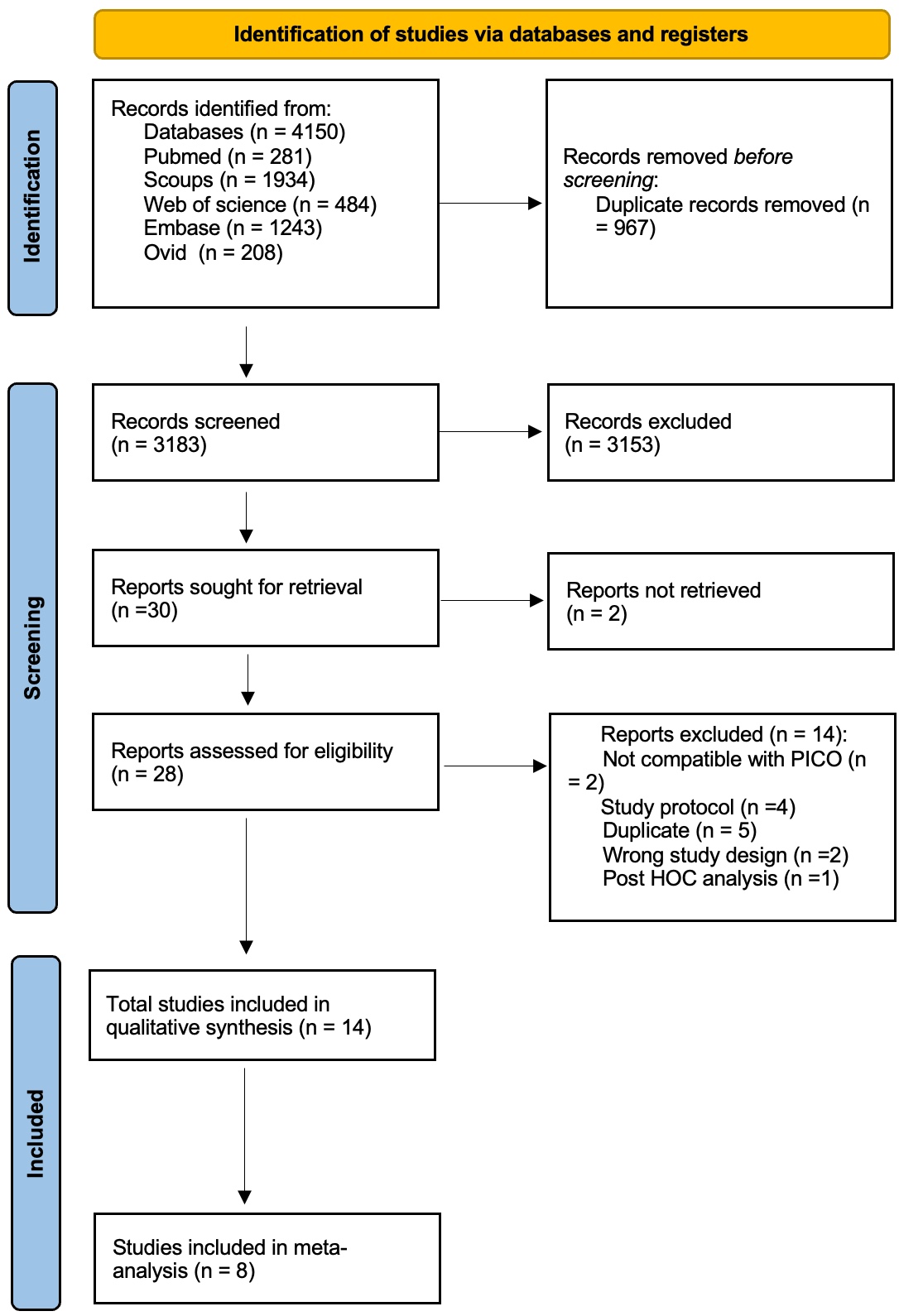 Figure (1) PRISMA Flow Chart Illustrating Search Strategy and Selection of Included Studies
Figure (1) PRISMA Flow Chart Illustrating Search Strategy and Selection of Included Studies
.jpg) Figure (2) Forest Plots of Treatment Effects for MTX in OA by Follow-Up Duration. (A) Pain Reduction (Fixed-Effects Model): Subgroups: < 6 months: Studies included: Enteshari-Moghaddam et al. 2019, Ferrero et al. 2021, Holanda et al. 2007, Kingsbury et al. 2024, Wenham 2013. 6 months: Studies included: Enteshari-Moghaddam et al. 2019, Kingsbury et al. 2024, Wang et al. 2023, Wenham 2013. 9 months: Studies included: Kingsbury et al. 2024. 12 months: Studies included: Ferrero et al. 2021, Kingsbury et al. 2024. Overall: Significant standardized mean difference (SMD) in pain reduction: -0.46 (95% CI: -0.64 to -0.27), with low heterogeneity (I2=19%). Test for overall effect: Z=4.89 (P < 0.00001). Test for subgroup differences: χ2=6.05, df=3 (P=0.11), I2=50.4%.
Figure (2) Forest Plots of Treatment Effects for MTX in OA by Follow-Up Duration. (A) Pain Reduction (Fixed-Effects Model): Subgroups: < 6 months: Studies included: Enteshari-Moghaddam et al. 2019, Ferrero et al. 2021, Holanda et al. 2007, Kingsbury et al. 2024, Wenham 2013. 6 months: Studies included: Enteshari-Moghaddam et al. 2019, Kingsbury et al. 2024, Wang et al. 2023, Wenham 2013. 9 months: Studies included: Kingsbury et al. 2024. 12 months: Studies included: Ferrero et al. 2021, Kingsbury et al. 2024. Overall: Significant standardized mean difference (SMD) in pain reduction: -0.46 (95% CI: -0.64 to -0.27), with low heterogeneity (I2=19%). Test for overall effect: Z=4.89 (P < 0.00001). Test for subgroup differences: χ2=6.05, df=3 (P=0.11), I2=50.4%.
(A) Pain Reduction (Random-Effects Model): Studies included: Same as fixed-effects model. Overall: Significant SMD: -0.47 (95% CI: -0.68 to -0.26), with a heterogeneity of (I2=19%). Test for overall effect: Z=4.33 (P < 0.0001). Test for subgroup differences: χ2=5.11, df=3 (P=0.16), I2=41.3%.
(B) WOMAC Improvement (Fixed-Effects Model): Subgroups: < 6 months: Studies included: Enteshari-Moghaddam et al. 2019, Ghosh 2020, Holanda et al. 2007, Kingsbury et al. 2024. 6 months: Studies included: Enteshari-Moghaddam et al. 2019, Kingsbury et al. 2024. 9 months: Studies included: Kingsbury et al. 2024. 12 months: Studies included: Kingsbury et al. 2024. Overall: Significant SMD in WOMAC improvement: -0.54 (95% CI: -0.70 to -0.39), with Considerable heterogeneity (I2=90%). Test for overall effect: Z=6.89 (P < 0.00001). Test for subgroup differences: χ2=12.26, df=3 (P=0.007), I2=75.5%. (C) Stiffness Reduction (Fixed-Effects Model): Subgroups: < 6 months: Studies included: Enteshari-Moghaddam et al. 2019, Holanda et al. 2007, Kingsbury et al. 2024, Strebkova 2024, Wenham 2013. 6 months: Studies included: Enteshari-Moghaddam et al. 2019, Kingsbury et al. 2024, Strebkova 2024, Wang et al. 2023, Wenham 2013. 9 months: Studies included: Kingsbury et al. 2024. 12 months: Studies included: Kingsbury et al. 2024. Overall: Significant SMD in stiffness reduction: -0.44 (95% CI: -0.65 to -0.23), with no heterogeneity (I2=0%). Test for overall effect: Z=4.05 (P < 0.0001). Test for subgroup differences: χ2=0.74, df=3 (P=0.86), (I2=0%) Pain, WOMAC and stiffness reduction showed consistent benefits across time points, with the largest effects at 6 months. Heterogeneity was negligible for stiffness and pain but substantial for WOMAC.
CI, confidence interval; SMD, standardized mean difference; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index; I2, heterogeneity index; df, degrees of freedom.
.jpg) Figure (3) Forest Plot of Adverse Events (AEs) Associated with MTX in Osteoarthritis. MAEs: Subgroup: Studies included: Ferrero et al. 2021, Kingsbury et al. 2024, Wang et al. 2023. Overall: Significant reduction in MAEs with MTX (RR = 0.77; 95% CI: 0.61–0.97), with no heterogeneity (I2=0%). Test for overall effect: Z=2.27 (P=0.02). SAEs: Subgroup: Studies included: Ferrero et al. 2021, Kingsbury et al. 2024, Wang et al. 2023. Overall: Non-significant reduction in SAEs with MTX (RR = 0.65; 95% CI: 0.31–1.34), with no heterogeneity (I2=0%). Test for overall effect: Z=1.17 (P=0.24). Total (MAEs + SAEs): Overall: Significant reduction in total AEs with MTX (RR = 0.75; 95% CI: 0.60–0.94), with no heterogeneity (I2=0%). Test for overall effect: Z=2.55 (P=0.01). Test for subgroup differences (MAE vs. SAE): χ2=0.20, df=1 (P=0.66), I2=0%. MTX demonstrated a significant reduction in total adverse events, driven primarily by MAEs. SAEs showed a non-significant trend toward reduction and also studies reported that SAEs were not related to MTX. No heterogeneity was observed across studies, and subgroup differences were negligible. CI, confidence interval; MAE, mild adverse event; SAE, serious adverse event; RR, risk ratio; I2, heterogeneity index; df, degrees of freedom.
Figure (3) Forest Plot of Adverse Events (AEs) Associated with MTX in Osteoarthritis. MAEs: Subgroup: Studies included: Ferrero et al. 2021, Kingsbury et al. 2024, Wang et al. 2023. Overall: Significant reduction in MAEs with MTX (RR = 0.77; 95% CI: 0.61–0.97), with no heterogeneity (I2=0%). Test for overall effect: Z=2.27 (P=0.02). SAEs: Subgroup: Studies included: Ferrero et al. 2021, Kingsbury et al. 2024, Wang et al. 2023. Overall: Non-significant reduction in SAEs with MTX (RR = 0.65; 95% CI: 0.31–1.34), with no heterogeneity (I2=0%). Test for overall effect: Z=1.17 (P=0.24). Total (MAEs + SAEs): Overall: Significant reduction in total AEs with MTX (RR = 0.75; 95% CI: 0.60–0.94), with no heterogeneity (I2=0%). Test for overall effect: Z=2.55 (P=0.01). Test for subgroup differences (MAE vs. SAE): χ2=0.20, df=1 (P=0.66), I2=0%. MTX demonstrated a significant reduction in total adverse events, driven primarily by MAEs. SAEs showed a non-significant trend toward reduction and also studies reported that SAEs were not related to MTX. No heterogeneity was observed across studies, and subgroup differences were negligible. CI, confidence interval; MAE, mild adverse event; SAE, serious adverse event; RR, risk ratio; I2, heterogeneity index; df, degrees of freedom.
To cite this abstract in AMA style:
Abdelsalam M, Lasheen M, Hafez H, Badwy B, El Sedafy O, Awad M. Safety, Short- and Long-Term Efficacy of Methotrexate in Osteoarthritis: A Systematic Review and Meta-Analysis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/safety-short-and-long-term-efficacy-of-methotrexate-in-osteoarthritis-a-systematic-review-and-meta-analysis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-short-and-long-term-efficacy-of-methotrexate-in-osteoarthritis-a-systematic-review-and-meta-analysis/
