Session Information
Date: Sunday, October 26, 2025
Title: (0280–0305) Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: The mevalonate (MVK) pathway synthesizes isoprenoids that mediate post-translational protein modifications via prenylation. Deficient protein prenylation due to decreased mevalonate kinase (MK) activity leads to innate immune activation and autoinflammatory disorders. Patients using statins, HMG-CoA reductase inhibitors, may present with myopathy. However, the precise pathogenesis of prenylation deficiency-associated inflammation and myotoxicity remains unclear. We identified a rare homozygous pathogenic variant in the HMGCS1 gene (c.265C >T, p. Arg89Trp), encoding for HMG-CoA synthase, the first enzyme in the MVK pathway, in two siblings presenting with necrotizing myopathy, arthropathy/arthritis, and susceptibility to infections.
Methods: Single-cell RNA sequencing (scRNA-seq) was conducted on PBMCs obtained from affected siblings, an unaffected sibling (wild-type), the mother (heterozygous) and unrelated healthy control. Muscle biopsies from affected siblings were used for bulk RNA sequencing. Using spectral flow cytometry, whole blood and/or PBMCs from affected individuals were assessed for IFN-g, IL-1b and IL-6 expression in monocytes, while IFN-g, granzyme B and perforin were assessed on NK and T cells.
Results: ScRNA-seq data evidenced an expansion of activated monocyte and cytotoxic populations in the affected but not in the unaffected brother, suggesting monocytes, T and NK cells as drivers of inflammation. Since muscle biopsies of the affected brothers revealed a strong IFN-g signature and IL-1 and IL-6 are crucial for inflammasome formation, we investigated these cytokines and inflammatory markers in LPS-stimulated myeloid subsets, and IL-12-stimulated NK cells and T cells of affected and unaffected controls. Percentage of IL-1b+ and IL-6+ non-classical monocytes (NCM) were significantly elevated in the affected siblings. IFN-g levels were increased in all monocyte subpopulations of the affected siblings, consistent with the IFN-g signature observed in the muscle biopsies. Monocytes expressing inflammatory markers, such as CD64, Siglec-1, and CD11c, were increased with a marked expression in NCM. Classical monocytes (CM), intermediate monocytes (IM), and NCM of the affected siblings presented lower PSGL-1 levels, aligned with the observed inflammatory responses, suggesting an infiltration of activated monocytes into muscle tissues. We observed a decrease in NK cells in affected donors, while T cells, specifically Th1 and Th17 cells, were expanded. Further, T cells presented elevated IFN-g expression in affected siblings, correlating with T cells infiltration seen in muscle tissue. Notably, T cells of the affected donors showed higher expression of granzyme B and perforin but decreased CD107a, indicating these cells were activated but with diminished degranulation activity.
Conclusion: Our data provide strong evidence that monocytes and T cells may play a pivotal role in the pathogenesis of inflammation and necrotizing myositis in patients with HMGCS1 deficiency, with NCM and cytotoxic T cells presenting the most proinflammatory profile suggesting an important role of these cells in sustaining HMGCS1 mutation-associated autoinflammatory disorder.
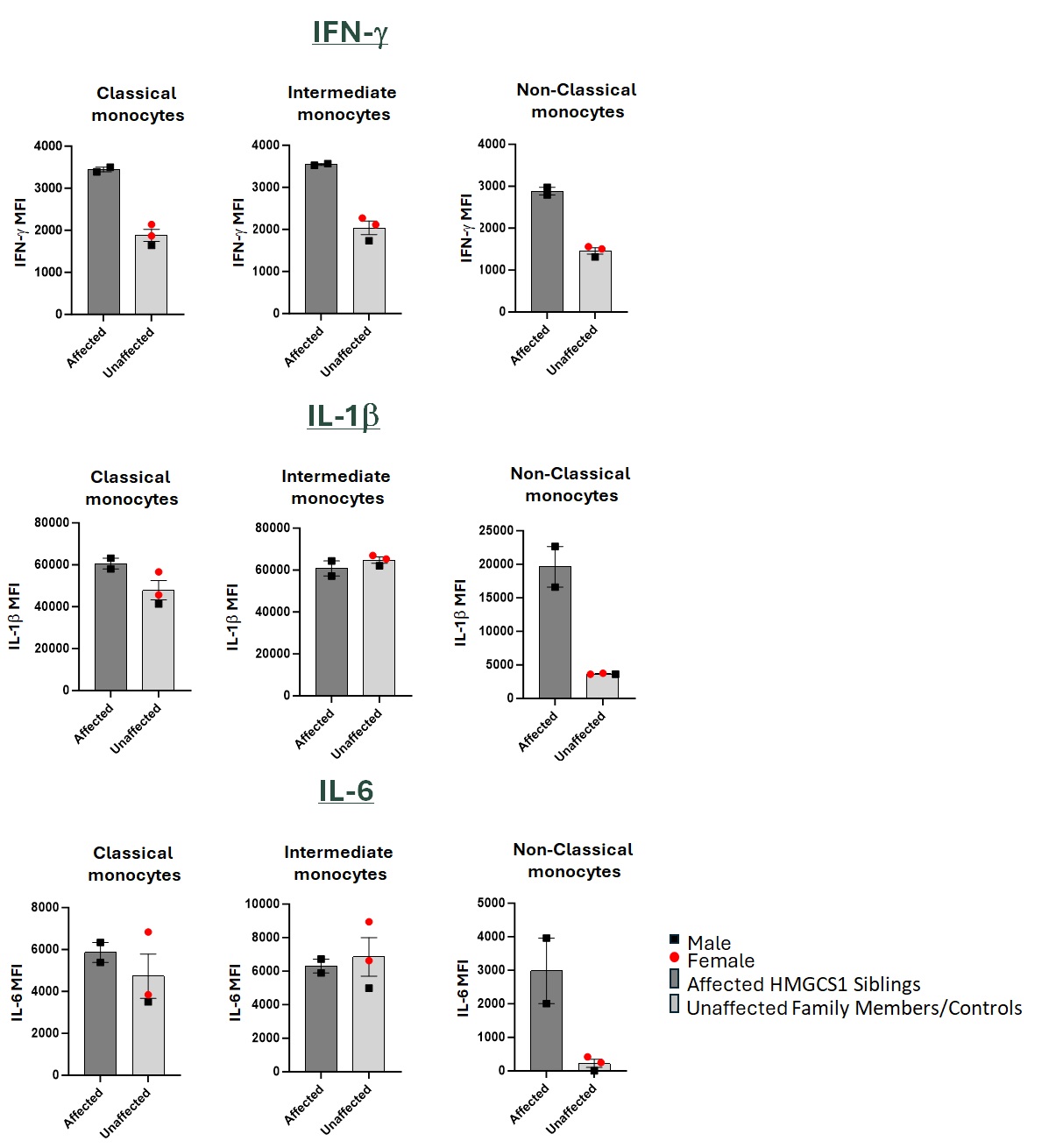 IFN-g, IL-1 and IL-6 release is increased in non-classical monocytes of HMGCS1 homozygous siblings.
IFN-g, IL-1 and IL-6 release is increased in non-classical monocytes of HMGCS1 homozygous siblings.
To cite this abstract in AMA style:
Helmold Hait S, Maclean M, Phung C, wigerblad G, Pinal Fernandez I, Esperanzate C, Wang h, Mammen A, Gahl w, Kastner D, Dell'orso S, Novacic D, Gadina m, Aksentijevich I. Novel HMGCS1 Deficiency Expands Proinflammatory Monocytes and Cytotoxic Populations with Increased Cytokine Release [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/novel-hmgcs1-deficiency-expands-proinflammatory-monocytes-and-cytotoxic-populations-with-increased-cytokine-release/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/novel-hmgcs1-deficiency-expands-proinflammatory-monocytes-and-cytotoxic-populations-with-increased-cytokine-release/
