Session Information
Date: Sunday, October 26, 2025
Title: (0233–0279) Miscellaneous Rheumatic & Inflammatory Diseases Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Interleukin 1 receptor associated kinase 4 (IRAK4) degradation may modulate toll-like receptor signaling and alleviate symptoms in rheumatoid arthritis and atopic dermatitis.1,2 BGB-45035, an orally administered CDAC, targets IRAK4 and in animal models, demonstrated a PK/PD association with suppression of IL-33–induced skin inflammation.This first-in-human study of BGB-45035 has 6 parts, data from parts A–C are reported. Parts A (single ascending dose [SAD]) and B (multiple ascending dose [MAD]) were in healthy participants; part C assessed MAD in healthy Chinese participants (China only). Objectives were to assess the safety, tolerability, PK, and PD of BGB-45035.
Methods: Participants were aged 18-55 years, with a body mass index of 18-32 kg/m2. Parts A-C were double-blind and placebo controlled. Part A (SAD) comprised 6 cohorts (X, 3X, 6X, 12X, 18X, 30X dosages); in each cohort, 6 participants received BGB-45035, and 2 participants received placebo. Participants were randomized (5:1) to BGB-45035 or placebo. Parts B and C (MAD) comprised 4 cohorts (X, 2X, 4X, 6X dosages) and 3 cohorts (2X, 4X, 6X dosages), respectively. Participants in both parts were randomized (3:1) to BGB-45035 or placebo once daily (QD) for 14 days. Adverse events and serious adverse events (SAEs) were monitored. PK parameters were estimated from plasma concentration data. PD assessments included IRAK4 protein concentration extracted from whole blood and cytokine production from ex vivo stimulated from whole blood.
Results: As of April 7, 2025, 80 participants (all men) were enrolled in parts A (n=48; median age 29 [range, 20-54] years) and B (n=32; median age 28 [range, 19-54] years) and 36 were enrolled in part C (median age 27 [range, 18-44] years). All active doses were generally well tolerated. No dose-dependent toxicity was observed; no safety signals were identified. Upper respiratory tract infection was the most common treatment-emergent adverse event, all were mild in severity (Table 1). One SAE, delirium, was considered severe but not treatment-related, and led to treatment discontinuation. Dose-dependent PK increases were observed with doses of X-30X, with maximum concentration observed at a median of 4 hours and a mean terminal half-life (t1/2) ranging from 58.5 to 95.6 hours across dosage groups (Table 2). Consistent with t1/2, a ≈5-6–fold increase in exposure was observed on day 14 with BGB-45035 QD dosing. Substantial IRAK4 degradation was observed via whole blood assay after BGB-45035 dosing (Figure 1).
Conclusion: BGB-45035 is a potent IRAK4 degrader that was generally well tolerated in healthy participants, with a favorable safety and PK profile over a 14-day dosing period. This supports continued evaluation of BGB-45035 as a therapy for autoimmune diseases.References:1. Ackerman L, et al. Nat Med. 2023;29(12):3127-3136.2. Lavazais S, et al. Sci Transl Med. 2023;15(683):eabj3289.
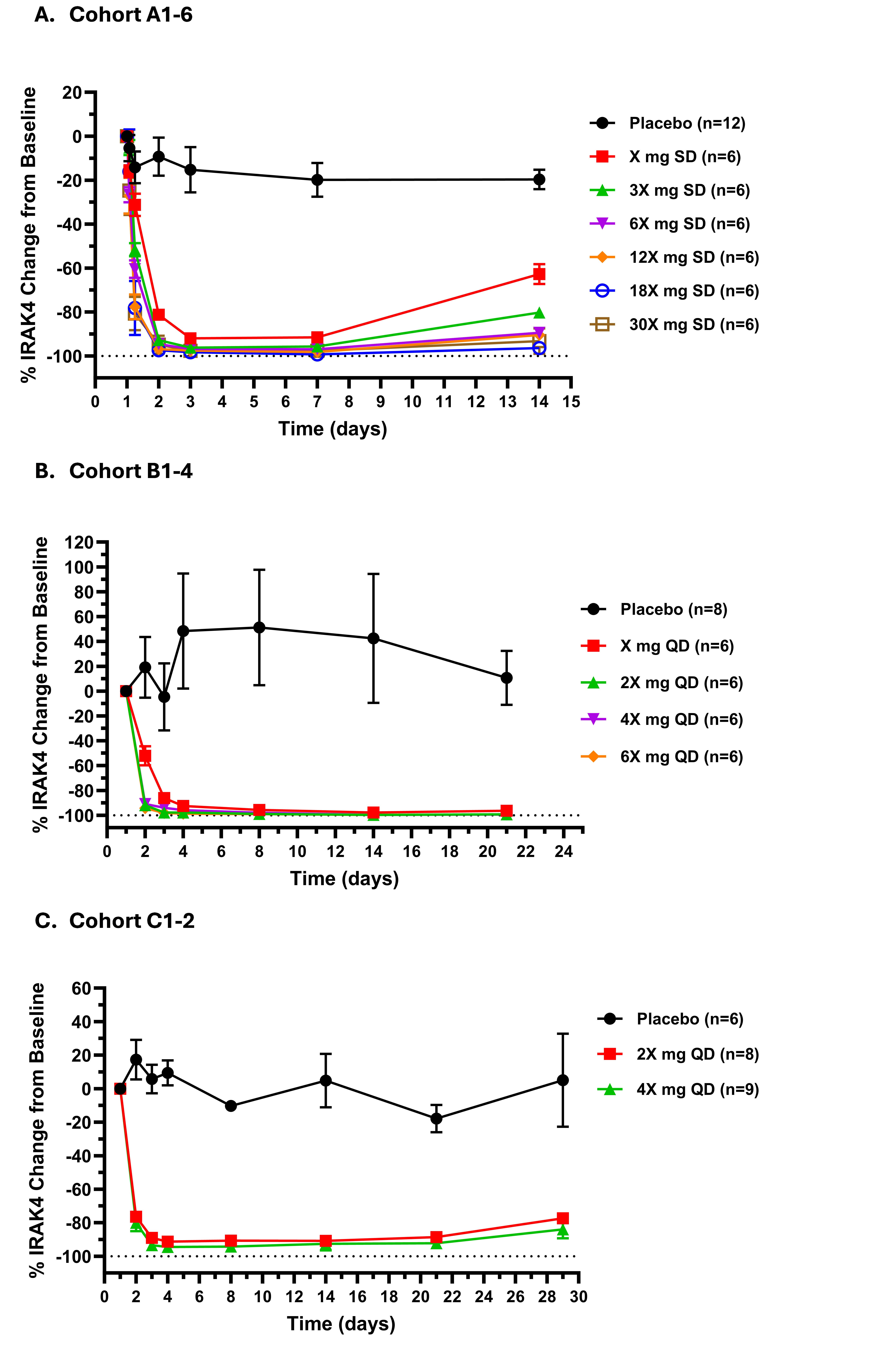 Table 1. TEAEs Occurring in ≥3 Participants by Preferred Term (Parts A-C; Safety Analysis Set [Nf116])
Table 1. TEAEs Occurring in ≥3 Participants by Preferred Term (Parts A-C; Safety Analysis Set [Nf116])
.jpg) Table 2. BGB-45035 Geometric Mean (CV% GM) PK Parameters After Single- and Multiple-Dose Administration
Table 2. BGB-45035 Geometric Mean (CV% GM) PK Parameters After Single- and Multiple-Dose Administration
.jpg) Figure 1. Mean Percent Reduction of IRAK4 in Part A-C
Figure 1. Mean Percent Reduction of IRAK4 in Part A-C
To cite this abstract in AMA style:
Mundra V, Yu C, Morley R, Yuan Y, Qin Y, Schneider J, Chen S, Zhou Z, Yao Z. A Phase 1, Randomized Study to Evaluate the Safety, Tolerability, Pharmacokinetics (PK), and Pharmacodynamics (PD) of Single and Multiple Ascending Doses and Food Effects of BGB-45035, a chimeric degradation activating compound (CDAC), in Healthy Participants [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/a-phase-1-randomized-study-to-evaluate-the-safety-tolerability-pharmacokinetics-pk-and-pharmacodynamics-pd-of-single-and-multiple-ascending-doses-and-food-effects-of-bgb-45035-a-chimeric-degr/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-phase-1-randomized-study-to-evaluate-the-safety-tolerability-pharmacokinetics-pk-and-pharmacodynamics-pd-of-single-and-multiple-ascending-doses-and-food-effects-of-bgb-45035-a-chimeric-degr/
