Session Information
Date: Sunday, October 26, 2025
Title: (0233–0279) Miscellaneous Rheumatic & Inflammatory Diseases Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Ocular involvement is a potential severe complication of Behçet’s Disease (BD). The traditional classification, by the International Study Group (ISG, 1990) requires the mandatory presence of recurrent painful oral ulcers, whereas the International Criteria for BD (ICBD, 2013) uses a scoring system without mandatory oral ulcers. Our objective was to assess the frequency and type of ocular manifestations accordingly to these classification criteria and compare the sensitivity and concordance of ISG versus ICBD, focusing on the impact of including or excluding ophthalmologic involvement and highlighting therapeutic implications.
Methods: Observational study of 142 BD, diagnosed by expert rheumatologists in Northern Spain (January 1980-November 2023). ISG and ICBD criteria were applied to these patients. Ocular manifestations included anterior, intermediate and posterior uveitis, panuveitis, dry eye, scleritis, episcleritis, and peripheral ulcerative keratitis (PUK). Proportions for each ocular manifestation were compared between the two classification sets, and p-values were provided to assess statistical significance. We calculated proportions for each ocular feature, assessed statistical significance (p-values), and evaluated sensitivity and concordance (Cohen’s Kappa, Prevalence and Bias Adjusted Kappa – PABAK) when ocular involvement was included or excluded. ICBD criteria showed greater sensitivity than ISG, specially due to the absence of oral ulcers in certain patients. Classifying these additional patients by ICBD facilitated the initiation of on-label biologic treatments (e.g., monoclonal anti-TNF), potentially improving the management of BD, particularly for those with ocular involvement and without oral ulcers.
Results: We studied 142 patients diagnosed as BD by expert rheumatologists, 84 met the ISG criteria while 116 met the ICBD criteria. Therefore, there were 32 additional patients with ICBD (Table 1). Among ISG-classified patients (n=84), ocular lesions were present in 49 patients (58.3%), while among ICBD-classified patients (n=116), 55 patients (47.4%) had ocular involvement (p=0.13). The frequency of uveitis (n=42; 50% ISG vs. n=47; 40.5% ICBD; p=0.18), and subtype (anterior, posterior, or panuveitis) were similar in both groups. With ocular inclusion, the non-adjusted Kappa was 0.405 (95% CI: 0.176–0.634), and the PABAK was 0.788, indicating strong agreement; without ocular inclusion, these values decreased to 0.443 (95% CI: 0.326–0.561) and 0.408 respectively, as shown on Table 2. Of the 32 patients classified as BD exclusively by ICBD, 7 were on anti-TNF therapy (3 infliximab, 4 adalimumab) and 2 on apremilast; 5 had uveitis and not oral ulcers with 1 receiving infliximab.
Conclusion: ICBD criteria showed greater sensitivity than ISG, specially due to the absence of oral ulcers in certain patients. Classifying these additional patients by ICBD facilitated the initiation of on-label biologic treatments (e.g., monoclonal anti-TNF), potentially improving the management of BD, particularly for those with ocular involvement and without oral ulcers.
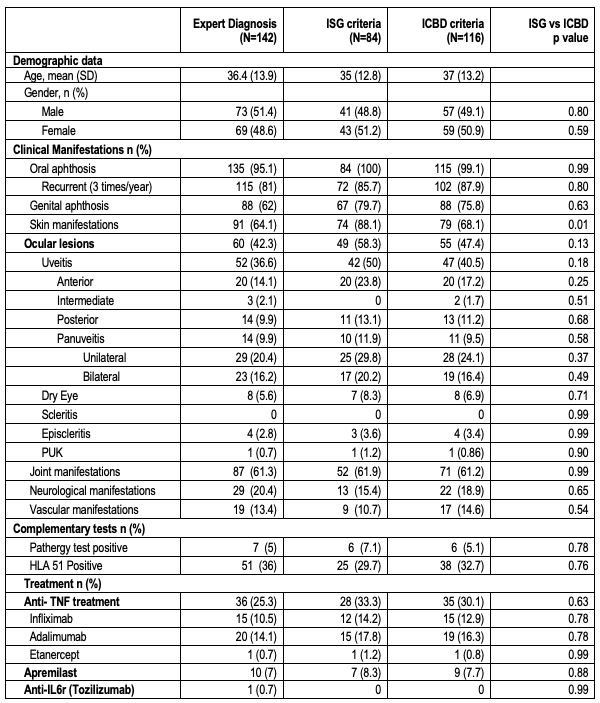 Table 1: Main clinical features according to different diagnosis criteria focusing on the ocular manifestations and biological treatments.
Table 1: Main clinical features according to different diagnosis criteria focusing on the ocular manifestations and biological treatments.
.jpg) Table 2: Metrics for Modified Classification Criteria: Concordance Analysis After the Addition or Exclusion of Ocular Manifestations in All Patients.
Table 2: Metrics for Modified Classification Criteria: Concordance Analysis After the Addition or Exclusion of Ocular Manifestations in All Patients.
To cite this abstract in AMA style:
Gálvez Sánchez R, Martín-Varillas J, Sánchez Bilbao L, Ferraz Amaro I, Aurrecoechea E, Blanco R. Ocular Involvement in Behçet’s Disease: Comparative Study of Two Classification Criteria in Clinical Practice [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/ocular-involvement-in-behcets-disease-comparative-study-of-two-classification-criteria-in-clinical-practice/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/ocular-involvement-in-behcets-disease-comparative-study-of-two-classification-criteria-in-clinical-practice/
