Session Information
Date: Sunday, October 26, 2025
Title: (0233–0279) Miscellaneous Rheumatic & Inflammatory Diseases Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Guselkumab (GUS), a dual-acting interleukin (IL)-23 inhibitor that potently neutralizes IL-23 and binds to CD64 (a receptor on cells that produce IL-23), is currently approved in the United States for treatment of ulcerative colitis (UC) and worldwide for the treatment of plaque psoriasis and psoriatic arthritis. While GUS has been shown to be safe in Crohn’s disease (CD) and UC, safety results have only been reported in individual trials to date. To characterize the overall safety profile of GUS in inflammatory bowel disease (IBD), we evaluated pooled safety data from Phase 2 and 3 clinical trials of GUS in CD and UC.
Methods: Participants with moderately to severely active CD (n=1492; GALAXI 1, 2, & 3, and GRAVITI studies) or UC (n=1514; QUASAR Induction Study 1, Induction Study 2, & Maintenance Study and VEGA-GUS monotherapy arm only) were assigned to GUS 200 mg intravenous (IV) or 400 mg subcutaneous (SC) induction (GRAVITI only) at Weeks 0, 4, and 8, followed by GUS 100 mg SC every 8 weeks or 200 mg SC every 4 weeks; or placebo (PBO). SC maintenance treatment continued through approximately 1 year. GUS studies were pooled for the induction period (GALAXI, GRAVITI, & QUASAR; IV and SC, Weeks 0-12 [VEGA excluded due to no PBO control]) and through 1 year (GALAXI, GRAVITI, QUASAR, & VEGA). In this pooled analysis, safety events were normalized to 100 participant-years (PY) of follow-up with corresponding confidence intervals.
Results: Through Week 12, 1703 participants were treated with GUS with 399.9 PY of follow-up. Rates of adverse events (AEs) were similar between participants treated with GUS (200 mg IV or 400 mg SC) and PBO (Figure 1A). AEs occurred at a rate of 202.82/100 PY and 223.26/100 PY, serious AEs at 11.75/100 PY and 28.56/100 PY, AEs leading to study agent discontinuation at 5.50/100 PY and 16.32/100 PY, and serious infections at 1.50/100 PY and 1.17/100 PY, for GUS and PBO, respectively. Through 1 year, 2057 participants were treated with GUS, with 1752.1 PY of follow-up. Rates of AEs, serious AEs, AEs leading to discontinuation, and serious infections, were no higher in GUS-treated than PBO-treated participants (Figure 1B). Through 1 year, no anaphylactic or serum sickness reactions were reported, and rates of malignancy, opportunistic infection, major adverse cardiovascular event, and clinically important hepatic disorders were low (Table 1). In GUS-treated participants, one participant (from an endemic region) reported active tuberculosis, and 2 deaths (acute myocardial infarction in a participant with pre-existing cardiovascular risk factors and non-suicidal gunshot wound, respectively) occurred.
Conclusion: Through 1 year of treatment, GUS demonstrated a favorable safety profile comparable to PBO in participants with CD and UC. No new safety concerns were identified.
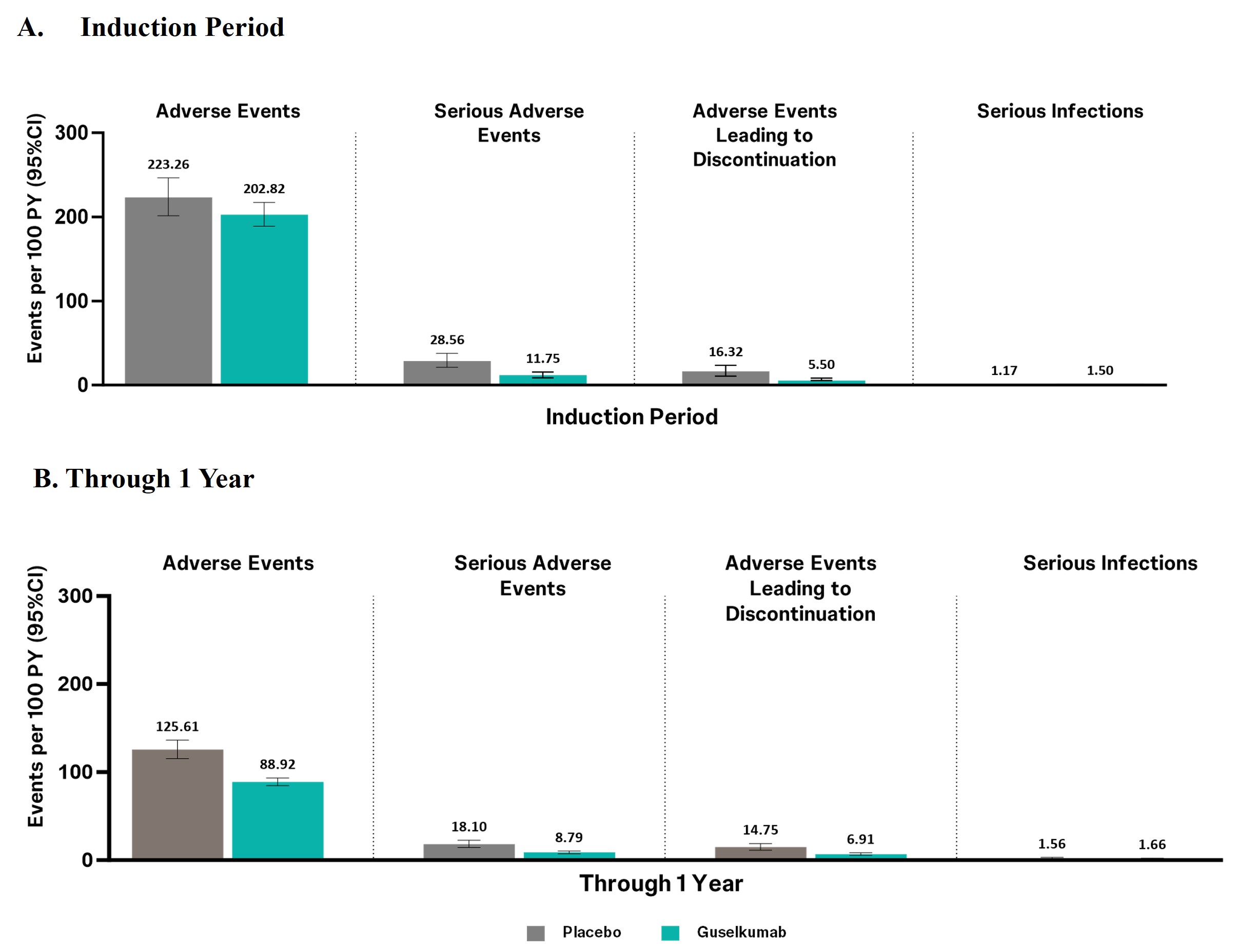 Figure 1. Key safety events in phase 2/3 IBD clinical studies during the induction period (Week 0-12) (A) and through 1 year (B)
Figure 1. Key safety events in phase 2/3 IBD clinical studies during the induction period (Week 0-12) (A) and through 1 year (B)
.jpg) Table 1: Targeted adverse events during the induction period and through 1 year; exposure-adjusted rates of events per 100 participant-years (PY) of follow-up and 95% confidence intervals (CI)
Table 1: Targeted adverse events during the induction period and through 1 year; exposure-adjusted rates of events per 100 participant-years (PY) of follow-up and 95% confidence intervals (CI)
To cite this abstract in AMA style:
Sands B, Panaccione R, Danese S, Panés J, Hisamatsu T, D’Haens G, Rampelbergh R, Olurinde M, Yee J, Lozenski K, Baker T, Yarandi S, Germinaro M, Vetter M, Li H, Ballina M, Allegretti J, Afzali A, Rubin D. Safety of Guselkumab in Inflammatory Bowel Disease Up to 1 Year: Integrated Safety Analysis of Phase 2 and 3 Studies in Crohn’s Disease and Ulcerative Colitis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/safety-of-guselkumab-in-inflammatory-bowel-disease-up-to-1-year-integrated-safety-analysis-of-phase-2-and-3-studies-in-crohns-disease-and-ulcerative-colitis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-of-guselkumab-in-inflammatory-bowel-disease-up-to-1-year-integrated-safety-analysis-of-phase-2-and-3-studies-in-crohns-disease-and-ulcerative-colitis/
