Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Arthrocentesis and joint injections are commonly performed to diagnose and to treat rheumatic conditions such as crystal-induced arthritis, septic arthritis, and inflammatory arthritis. While generally safe, these procedures carry risks of iatrogenic infection and bleeding (Courtney 2005, Dooley 2002), particularly in cancer patients, who often have leukopenia and thrombocytopenia from chemotherapy or are on anticoagulation therapy (Ahmed 2012). Despite proposed thresholds for prophylactic transfusions (e.g., 20,000 and 10,000/microliter) in more invasive procedures, specific guidelines on the safety of arthrocentesis in cancer patients remain lacking (Kaufman 2015, Avvisaati 2003).
Methods: We conducted a retrospective cohort study of patients who underwent arthrocentesis or joint injections between 2006 and 2016 at MD Anderson Cancer Center. Baseline demographic information, clinical characteristics (e.g. primary cancer diagnosis, smoking status, comorbidities and etc), and clinical outcomes were collected up to 12 weeks post-procedure. The primary outcome was bleeding, and the secondary outcome was iatrogenic infection. To evaluate the safety of arthrocentesis and joint injections in thrombocytopenic patients (platelet count ≤50,000/µL), we performed exact matching on sex, age, cancer type, anti-platelet, and anti-coagulant drugs use in a 1:2 ratio with patients with normal platelet count (≥140,000/µL) to compare the outcomes between the two patient groups.
Results: Among 95 arthrocentesis in thrombocytopenic patients and 760 in patients with normal platelet counts, no cases of bleeding or iatrogenic infection were observed in either group. After matching, thrombocytopenic patients (n=89) and controls (n=174) were comparable in demographic and clinical characteristics. Univariable analysis showed that initial visits to the ER and inpatient were associated with higher risk of septic arthritis. However, hemoglobin greater than the median and white blood cell greater than the median are associated with a lower risk of septic arthritis. Finally, thrombocytopenic patients had a higher risk of initial septic arthritis, while even adjusting for white blood count (OR 10.8; 95% CI: 2.9-39.8; P< 0.001).
Conclusion: Arthrocentesis and joint injections appear safe in cancer patients with thrombocytopenia, as no bleeding or iatrogenic infections were observed. However, the higher incidence of septic arthritis in thrombocytopenic patients underscores the importance of microbiological analysis of synovial fluid. These findings support the safety of these procedures while highlighting the need for vigilance in high-risk patients.
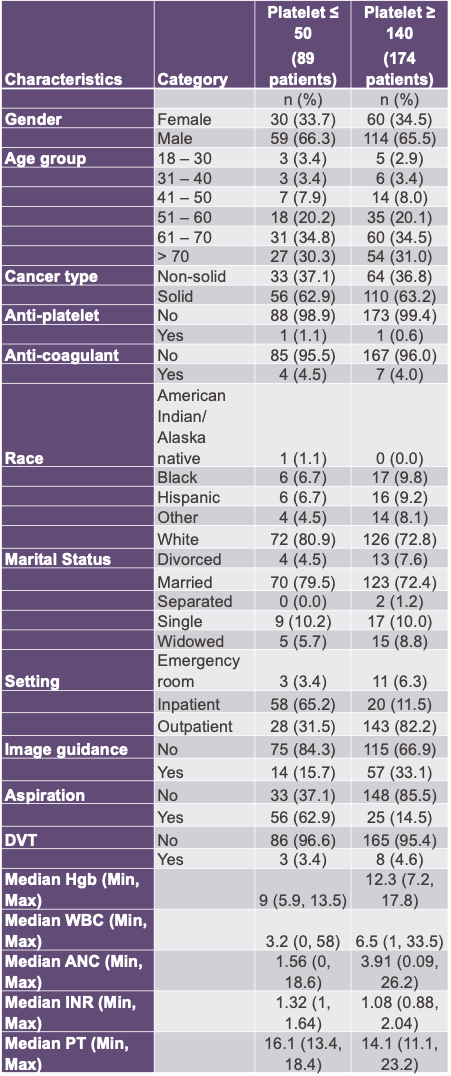 Demographics after exact matching 1:2. The absolute standardized differences were less than 10% for all 5 categories after exact matching, indicating good balances on the matching factors.
Demographics after exact matching 1:2. The absolute standardized differences were less than 10% for all 5 categories after exact matching, indicating good balances on the matching factors.
.jpg) Odds of septic arthritis vs. patient characteristics based on univariable logistic regression with 95% confidence interval
Odds of septic arthritis vs. patient characteristics based on univariable logistic regression with 95% confidence interval
.jpg) Odds of Septic Arthritis based on low platelet count and setting, hemoglobin, and WBC based on multivariable analysis with ⍺ at 0.05. Highlighted Odds Ratio have a p-value < 0.05.
Odds of Septic Arthritis based on low platelet count and setting, hemoglobin, and WBC based on multivariable analysis with ⍺ at 0.05. Highlighted Odds Ratio have a p-value < 0.05.
To cite this abstract in AMA style:
Li Y, Leung C, Lin H, Lopez-Olivio A, Tayar J. A Retrospective Safety Analysis based on Platelets in Arthrocentesis and Joint injections in Cancer Patients [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/a-retrospective-safety-analysis-based-on-platelets-in-arthrocentesis-and-joint-injections-in-cancer-patients/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-retrospective-safety-analysis-based-on-platelets-in-arthrocentesis-and-joint-injections-in-cancer-patients/
