Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Emerging evidence implicates neutrophil activation and neutrophil extracellular trap (NET) formation (i.e., NETosis) in amplifying APS-associated thromboinflammation. Gasdermin D (GSDMD), a substrate of inflammatory caspases that is best known for forming membrane pores during macrophage pyroptosis, can also support interleukin-1β (IL-1β) release and NETosis by neutrophils. Based on preliminary data demonstrating elevated inflammatory caspase activity in APS neutrophils, we investigated the potential role of neutrophil GSDMD in APS pathophysiology.
Methods: NETosis was measured with the SYTOX Green assay and/or citrullinated histone H3-based quantification. Cleaved, pore-forming N-terminal GSDMD (GSDMD-NT) was identified using flow cytometry, immunoblotting, and confocal microscopy. Caspase-1 activity was measured with a luminescent assay. Plasma IL-1β, IL-18, and calprotectin were quantified by ELISA. In vitro, neutrophils were treated with inhibitors of GSDMD (disulfiram, necrosulfonamide) or caspase-1 (VX-765), followed by exposure to IgG fractions from controls or APS patients or affinity-purified anti-β2GPI. GSDMD-deficient (Gsdmd-/-) mice were assessed in the context of APS IgG-accelerated venous thrombosis in the inferior vena cava (IVC).
Results: Compared with healthy controls (n=13), neutrophils from patients with primary APS (n=25) exhibited a 3-fold increase in caspase-1 activity (p< 0.0001), as well as a 4-fold higher formation of cleaved, pore-competent GSDMD-NT (p< 0.01). A 2.8-fold rise in plasma IL-18 levels (p< 0.01) was also appreciated in the same patient cohort. Notably, both neutrophil caspase-1 activity (r=0.52, p=0.0058) and plasma IL-18 (r=0.41, p=0.031) positively correlated with neutrophil GSDMD-NT, as did plasma calprotectin (r=0.64, p=0.0024), a biomarker of neutrophil activation and NET formation. In vitro, the exposure of control neutrophils to APS patient IgG significantly increased caspase-1 activity (2.5-fold, p< 0.05), GSDMD-NT formation (3-fold, p< 0.01), IL-1β secretion (3-fold, p< 0.01), and NETosis (3-fold, p< 0.0001). Confocal microscopy demonstrated that APS IgG triggered the preferential accumulation of GSDMD-NT in neutrophil plasma membranes. Furthermore, blocking GSDMD-NT cysteine-191, which is required for efficient pore formation, with either disulfiram or necrosulfonamide prevented neutrophil activation by APS IgG; the inhibition of caspase-1 with VX-765 was similarly protective. Compared with wild-type mice, Gsdmd⁻/⁻ mice (n=5) exhibited a 2-fold reduction in plasma IL-1β (p< 0.01, n=5) and developed significantly smaller thrombi in response to APS IgG-associated thrombosis (mean 3.1 mg versus 7.6 mg, p< 0.01).
Conclusion: By releasing IL-1β, NETs, and other inflammatory mediators, GSDMD pores may play a crucial role in linking innate immunity to immunothrombosis in APS (Figure 1). Since GSDMD deficiency and inhibition (with repurposed agents like disulfiram) reduce neutrophil activation in APS models, targeting GSDMD could offer a new approach for managing inflammation-driven thrombosis that may not respond to anticoagulation alone.
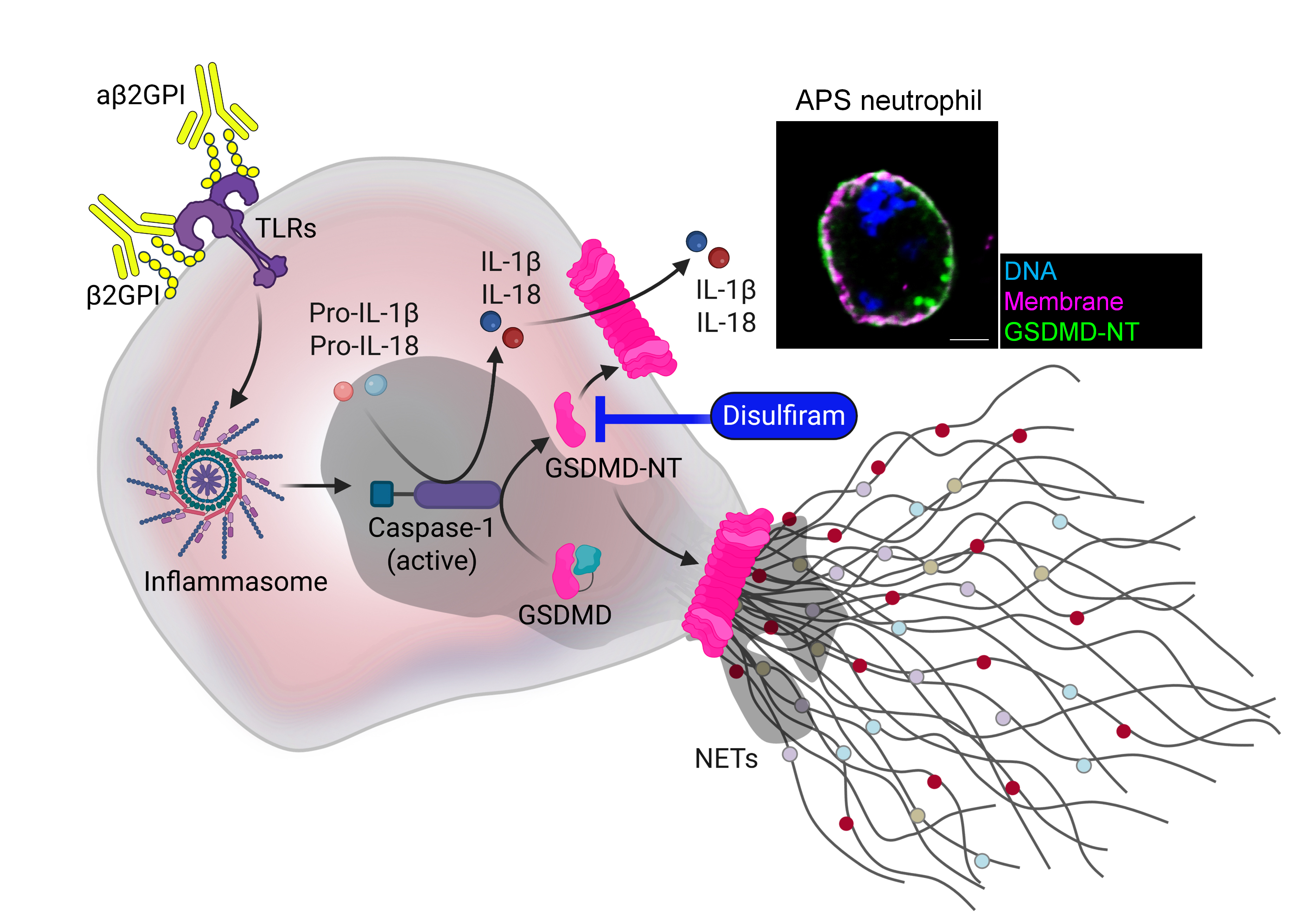 Figure 1. Schematic illustration depicting the role of active GSDMD in promoting the release of mature interleukins and NETs in APS neutrophils.
Figure 1. Schematic illustration depicting the role of active GSDMD in promoting the release of mature interleukins and NETs in APS neutrophils.
To cite this abstract in AMA style:
Somanathapura N, Newman T, Liu C, Yalavarthi S, Sarosh C, Madison J, Tambralli A, Zuo Y, Knight J. Neutrophil Gasdermin D Pores as Potential Therapeutic Targets in APS-Associated Thromboinflammation [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/neutrophil-gasdermin-d-pores-as-potential-therapeutic-targets-in-aps-associated-thromboinflammation/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/neutrophil-gasdermin-d-pores-as-potential-therapeutic-targets-in-aps-associated-thromboinflammation/
