Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Previous studies identified single nucleotide polymorphisms (SNPs) that affect hepatocyte and adipocyte response to glucocorticoids (GCs). We aimed to determine if these candidate SNPs impact the risk of metabolic adverse events (AEs) in patients with RA treated with GCs.
Methods: We genotyped participants from the Veterans Affairs RA (VARA) registry, a prospective cohort of RA, using the Illumina GSA v2. Imputation was performed using TopMED Imputation Server. We evaluated 22 candidate SNPs known to impact hepatocyte or adipocyte response to GCs. Among participants initiating a GC course (identified through prescription fill data), Cox proportional hazard models assessed associations between each SNP and composite metabolic AEs (incident diabetes, acute cardiovascular event, or sustained weight gain ≥3kg), censoring 90 days after GC discontinuation, end of follow-up, or death. Models were adjusted for sex, age, and principal components of genetic background and clustered by participant to account for multiple observations per patient. As a negative control, we repeated analyses among participants initiating a DMARD with no GC exposure in the preceding 180 days. Using the Benjamini-Hochberg false discovery rate (FDR), a p-value < 0.0021 was considered significant. SNPs with the strongest associations were screened in the Genotype-Tissue Expression Portal (GTEx) for expression quantitative trait loci (eQTL) in hepatic or adipose tissue to understand the effect of genetically regulated gene expression on the composite outcome. Two sample mendelian randomization (MR) was performed for the effect of rs6495481 on metabolic AEs.
Results: In 2597 VARA participants (88% male, mean age 68 years), there were 1559 prednisone initiations among 994 participants, and 3482 DMARD initiations among 1330 participants unexposed to GCs. Incidence of all metabolic AEs were higher in the prednisone initiation cohort (Table 1). In time to event analysis, no SNP reached experiment-wide significance for the primary or secondary outcomes (Fig. 1). The SNP rs6495481 C >T demonstrated the strongest negative association with composite AEs, driven by less sustained weight gain, but did not reach significance (composite OR 0.76, 95% CI 0.63, 0.93, p = 0.007, Fig. 1, Fig. 2). rs6495481 was not associated with composite AEs in the cohort unexposed to GCs. In GTEx, rs6495481 is an eQTL for fumarylacetoacetate hydrolase (FAH) in several tissues, including the liver. MR demonstrated a strong protective effect of downregulated hepatic gene expression of FAH on the risk of composite AEs following GC exposure (Wald ratio 3.74, SE 0.65, p = 9.0 x10-9).
Conclusion: Individual genetic variants impacting hepatocyte and adipocyte response to GCs did not meet the experiment-wide threshold for associations with the risk of metabolic AEs in patients with RA initiating prednisone. These analyses may have been underpowered to identify more modest associations. MR results suggest a potential biologic effect of these pathways on GC toxicity. Future studies should assess whether a combination of genetic factors can be combined with clinical factors to identify patients at high risk of metabolic AEs to guide clinical decision making.
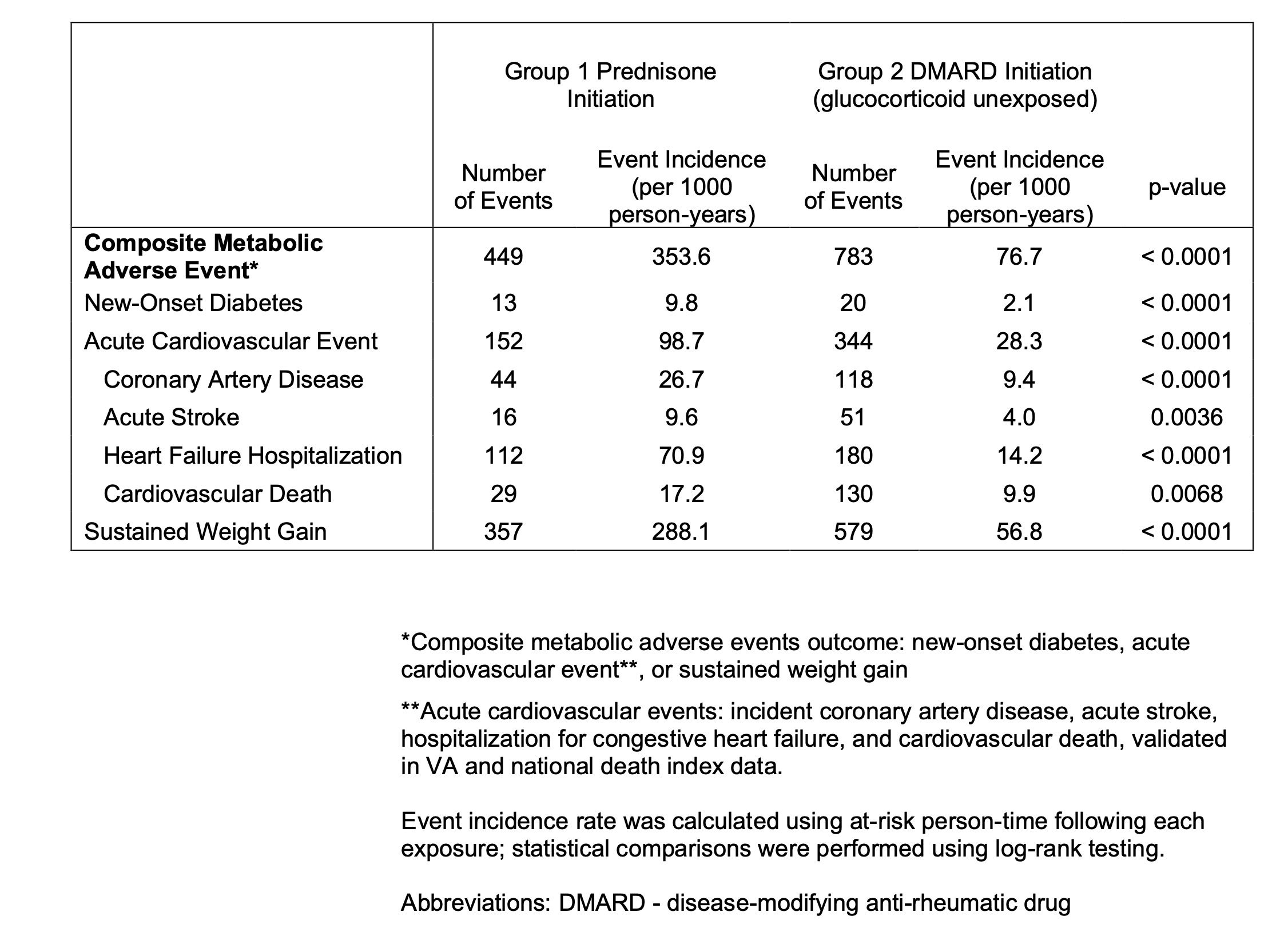 Table 1. Incidence of metabolic adverse events in patients with RA initiating prednisone compared to those unexposed to glucocorticoids initiating a disease-modifying anti-rheumatic drug.
Table 1. Incidence of metabolic adverse events in patients with RA initiating prednisone compared to those unexposed to glucocorticoids initiating a disease-modifying anti-rheumatic drug.
.jpg) Figure 1. Association of SNPs related to glucocorticoid metabolism with adverse metabolic outcomes in VARA.
Figure 1. Association of SNPs related to glucocorticoid metabolism with adverse metabolic outcomes in VARA.
Bold text indicate p-value < 0.05. No tests reached the experiment-wide significance threshold of 0.0021.
Values are hazard ratios from Cox regression model adjusting for age, sex, and population structure. Composite events include new-onset diabetes, acute cardiovascular event, and sustained weight gain. Cardiovascular events include coronary artery disease, acute stroke, congestive heart failure, cardiovascular death.
.jpg) Figure 2. Association of rs6495481 with adverse metabolic outcomes in VARA participants initiating glucocorticoids.
Figure 2. Association of rs6495481 with adverse metabolic outcomes in VARA participants initiating glucocorticoids.
Patients with one T allele at rs6495481 (chr15, 80500456, C>T) had lower risk of the composite metabolic adverse event, driven by lower risk for sustained weight gain, but results were not statistically significant at a threshold of p < 0.0021. Confidence intervals are not corrected for the FDR.
To cite this abstract in AMA style:
Riley T, England B, Wheeler A, Roul P, Cannon G, Sauer B, Kunkel G, Wysham K, Wallace B, Reimold A, Kerr G, Smith I, Richards J, Lee I, Lazar M, Hu W, Levin M, Damrauer S, Xiao R, Johnson T, Mikuls T, Baker J, George M. Genetic regulators of corticosteroid response in hepatic and adipose tissue and risk of adverse metabolic outcomes in patients with rheumatoid arthritis initiating glucocorticoids. [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/genetic-regulators-of-corticosteroid-response-in-hepatic-and-adipose-tissue-and-risk-of-adverse-metabolic-outcomes-in-patients-with-rheumatoid-arthritis-initiating-glucocorticoids/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/genetic-regulators-of-corticosteroid-response-in-hepatic-and-adipose-tissue-and-risk-of-adverse-metabolic-outcomes-in-patients-with-rheumatoid-arthritis-initiating-glucocorticoids/
