Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: PEGylated uricases have already demonstrated therapeutic modality in the treatment of refractory gout patients. PRX-115 is a recombinant homotetrameric uricase enzyme, produced from Candida utilis using a proprietary plant cell-based expression system. The enzyme is uniquely modified post purification with exclusive polyethylene glycol (PEG) molecules. PRX-115 is designed to have high specific activity, increased stability and reduced immunogenic potential.
Methods: A first-in-human single ascending dose, double-blinded, study (FIH-SAD, NCT05745727) was conducted to assess the safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of PRX-115 following a single intravenous (IV) infusion. The study recruited 64 participants, largely with elevated urate ( >6.0 mg/dL), randomized to PRX-115 or placebo in a 3:1 ratio (8 cohorts; 6 participants on active and 2 on placebo per cohort). Participants were followed for 85 days (12 weeks). Data from the initial seven cohorts (total of 56 participants) is presented below (data from the complete study will be presented at the conference).
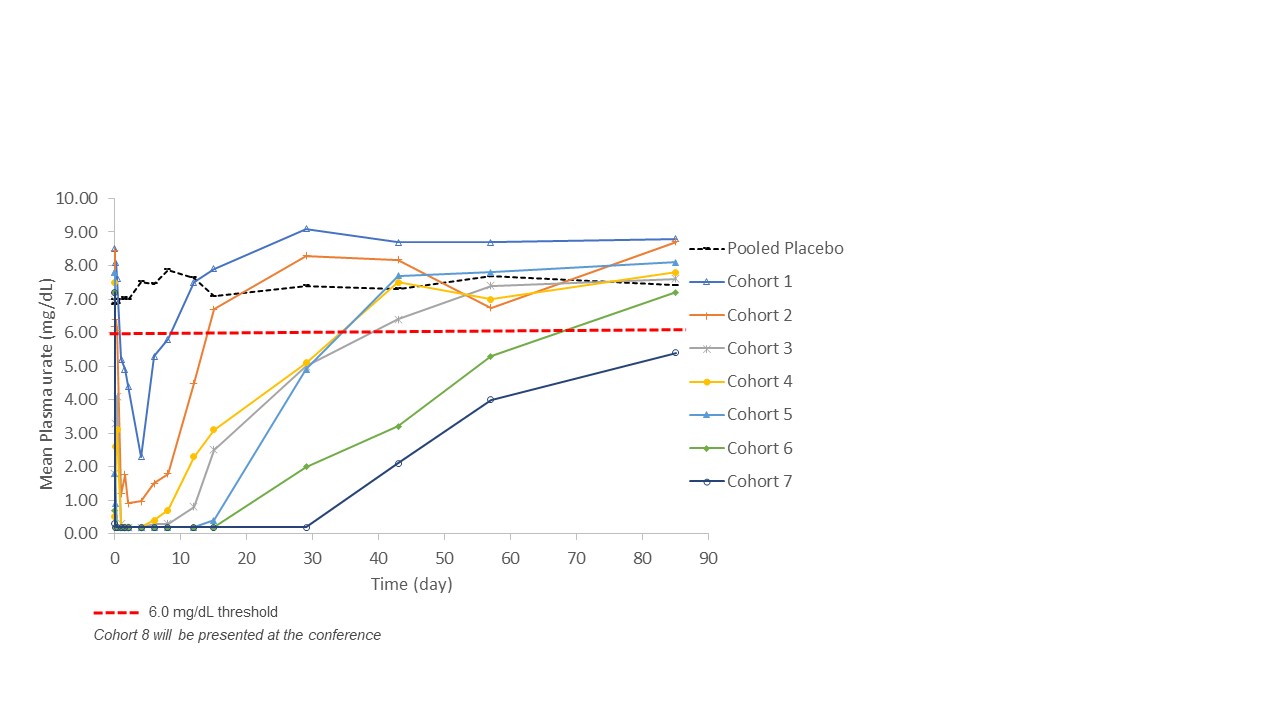
Results: Key results from the initial seven cohorts indicate that single ascending doses of PRX-115 were generally well-tolerated. Overall, 11 (26%) participants treated with PRX-115 reported a total of 18 related Treatment Emergent Adverse Events (TEAEs), the majority being mild to moderate and transient in nature. One serious adverse event (SAE, anaphylactic reaction) occurred at a low dose, immediately following the commencement of the infusion. The SAE was fully resolved and the participant continued in the study. PRX-115 exposure increased in a dose–dependent manner with maximal concentrations observed, in general, immediately post-infusion, and with quantifiable concentrations up to 85 days (12 weeks) post dosing at the high doses. In all tested doses, a single dose of PRX-115 rapidly reduced plasma urate below 6.0 mg/dL. The urate-lowering effect and the duration of the response were dose-dependent, with urate concentrations below 6.0 mg/dL that lasted for 12 weeks (Figure 1).
Conclusion: The results from the initial seven cohorts of this FIH-SAD study demonstrate that PRX-115 has the potential to be a promising treatment option for patients with gout, and may be able to offer a wide dosing interval. Further studies are warranted to confirm these findings and to establish the long-term safety and efficacy of PRX-115 in gout patients.
To cite this abstract in AMA style:
Schwabe C, Cohen Barak O, Cole A, Reuveni H, Shelev L, Blinder-Haddad L, Dalbeth N. Prolonged Plasma Urate-Lowering After a Single Intravenous Administration of PRX-115, a Novel PEGylated Uricase, in Participants with Elevated Urate Levels [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/prolonged-plasma-urate-lowering-after-a-single-intravenous-administration-of-prx-115-a-novel-pegylated-uricase-in-participants-with-elevated-urate-levels/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/prolonged-plasma-urate-lowering-after-a-single-intravenous-administration-of-prx-115-a-novel-pegylated-uricase-in-participants-with-elevated-urate-levels/