Session Information
Date: Tuesday, November 19, 2024
Title: Abstracts: Muscle Biology, Myositis & Myopathies – Basic & Clinical Science II
Session Type: Abstract Session
Session Time: 11:00AM-12:30PM
Background/Purpose: Several case reports and case series of idiopathic inflammatory myositis (IIM) following COVID-19 vaccination have been reported. This study evaluated the association of prior COVID-19 vaccination with the development of IIM in US Veterans enrolled in the Veterans Health Administration (VHA).
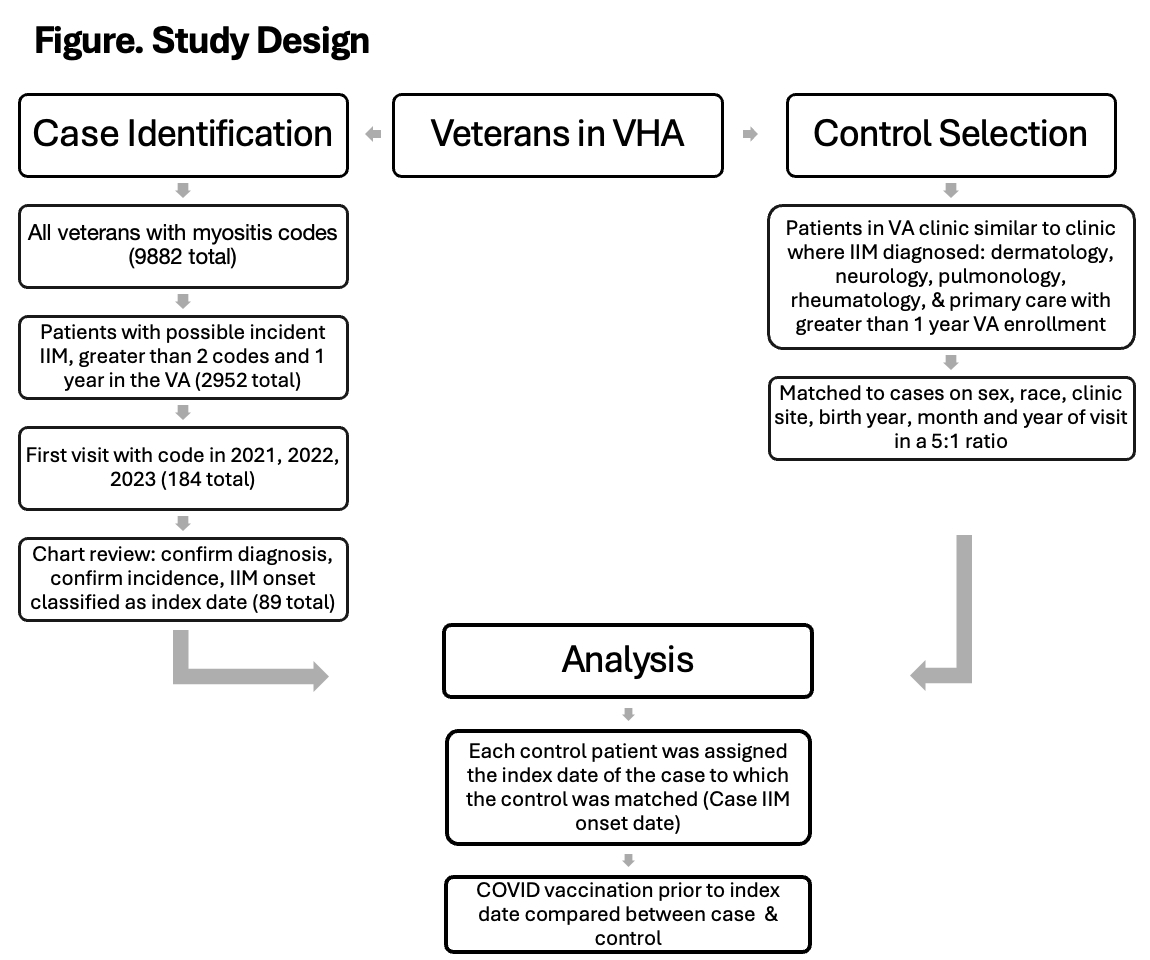
Methods: This case-control study evaluated newly diagnosed cases of IIM between January 1, 2021, and March 1, 2024, in the VHA. Potential new onset IIM cases included Veterans who received at least two ICD myositis codes during 2021-2023 after at least one year of entering the VHA (Figure). The electronic medical records of the cases identified as potential new onset IIM were reviewed 1) to determine if case met 2017 EULAR/ACR criteria for IIM, 2) confirm that the case was an incident case during the study period, and 3) record the date of IIM onset (index date). Data on clinical features and myositis antibodies were also collected. Each IIM incident case was matched 1:5 to control patients on gender, race, birth year, clinic site where IIM diagnosis first recorded, and month and year of clinic visit with first ICD code diagnosis. VHA corporate data warehouse (CDW) provided demographic, VHA care duration, and COVID-19 vaccination data.
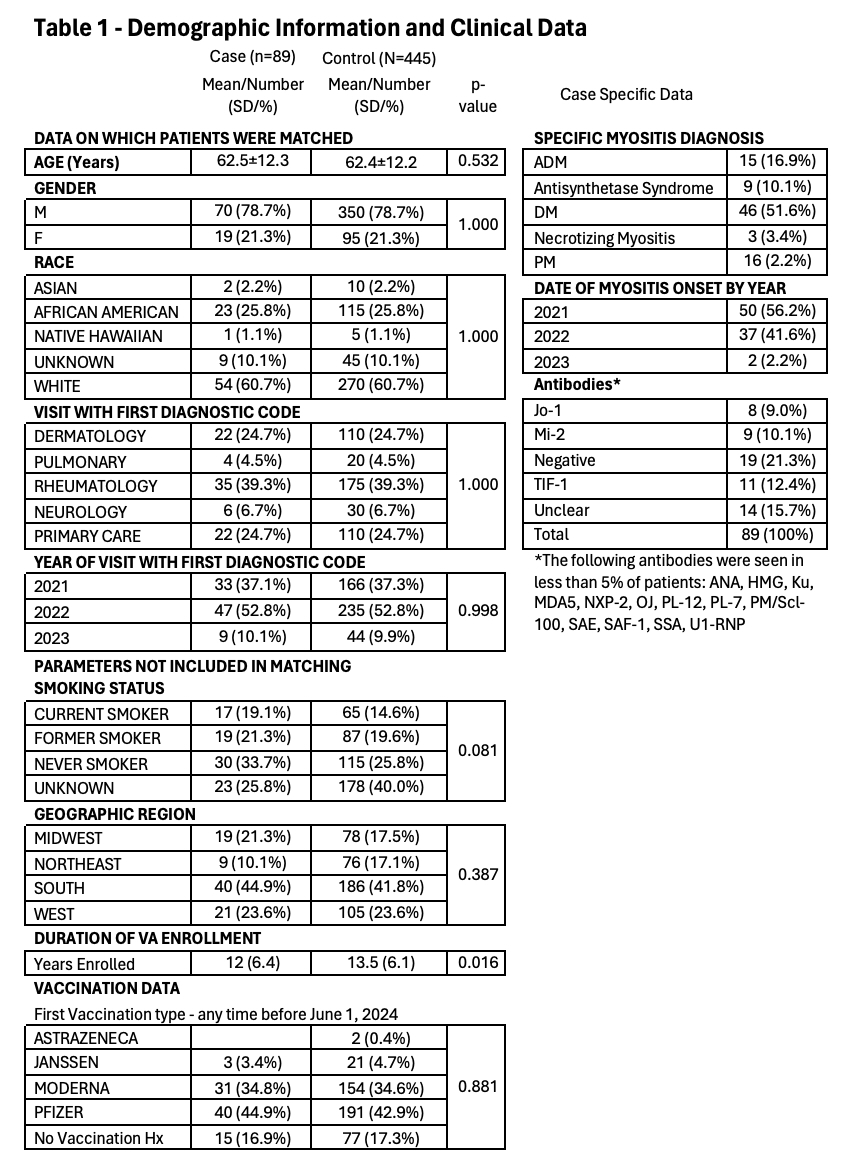
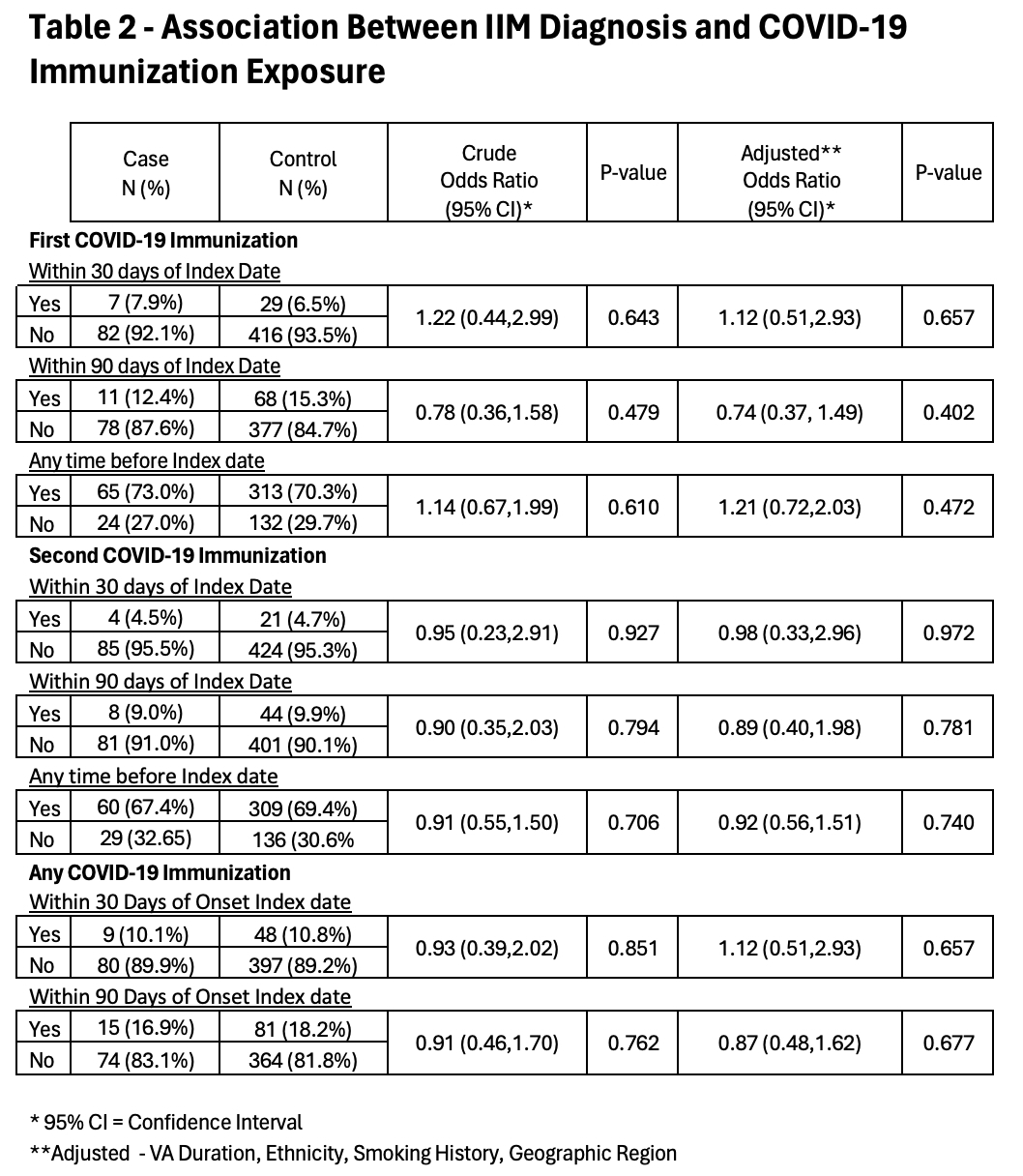
Results: Of the 184 potential cases, 89 patients were confirmed to be incident IIM cases meeting our inclusion criteria and were matched to 445 controls (Figure, Table 1). Classic dermatomyositis was the most common IIM classification, 46 (51.6%), followed by polymyositis, 16 (18%), and amyopathic dermatomyositis, 15 (16.9%). The most common antibody identified was TIF-1, 11 (12.4%), followed by Mi-2, 9 (10.1%), and Jo-1, 8 (9.0%). Patients were predominately white males and in their sixth decade of life. However, 25.8% were African Americans and 21.3% were females; 30% were current or former smokers, and the average length of enrollment in the VA was 12 years. There were 7 (7.9%) case patients and 29 (6.5%) control patients who received their first vaccination within 30 days before the index date (OR 1.22, p=0.643, adjusted OR 1.12, p=0.657) (Table 2) and 11 (12.4%) case patients and 68 (15.3%) control patients who received their first vaccination within 90 days of the index date (OR 0.78, p=0.479, adjusted OR 0.74, p=0.402). All other comparisons also failed to identify a statistically significant association of COVID-19 vaccination and IIM (Table 2).
Conclusion: This study is the first to compare the risk of developing myositis after receiving the COVID-19 vaccination compared to a control population. While the potential for small risk could not be identified by this case-control study, there appears to be no major increased risk for the development of an IIM after COVID-19 vaccination. Confirmation of these findings by additional studies will be helpful in addressing this association. These data provide useful information for discussion with patients when considering the risk and benefits of COVID-19 vaccination.
To cite this abstract in AMA style:
Hernandez C, Schlesinger N, Rojas J, Walsh J, Braaten T, Kunkel G, Jones M, brian S, Facelli J, Cannon g, Lebiedz-Odrobina D. The Risk for Development of Myositis Is Not Increased After COVID-19 Vaccination Among U.S. Veterans [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/the-risk-for-development-of-myositis-is-not-increased-after-covid-19-vaccination-among-u-s-veterans/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-risk-for-development-of-myositis-is-not-increased-after-covid-19-vaccination-among-u-s-veterans/