Session Information
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: High ferritin is an important and sensitive biomarker for the diverse and deadly group of cytokine storm syndromes grouped together under the term hemophagocytic lymphohistiocytosis (HLH). Early identification of the syndrome and its contributors are critical to guiding targeted treatments and preventing immunopathology, morbidity, and mortality. We lack specific diagnostic biomarkers, which complicates etiologic work-up and delays targeted intervention. Through implementing a hyperferritenemia alert system, we hoped to identify the landscape of hyperferritinemic patients, collect the earliest samples for research purposes, and test these samples for relevant biomarkers.
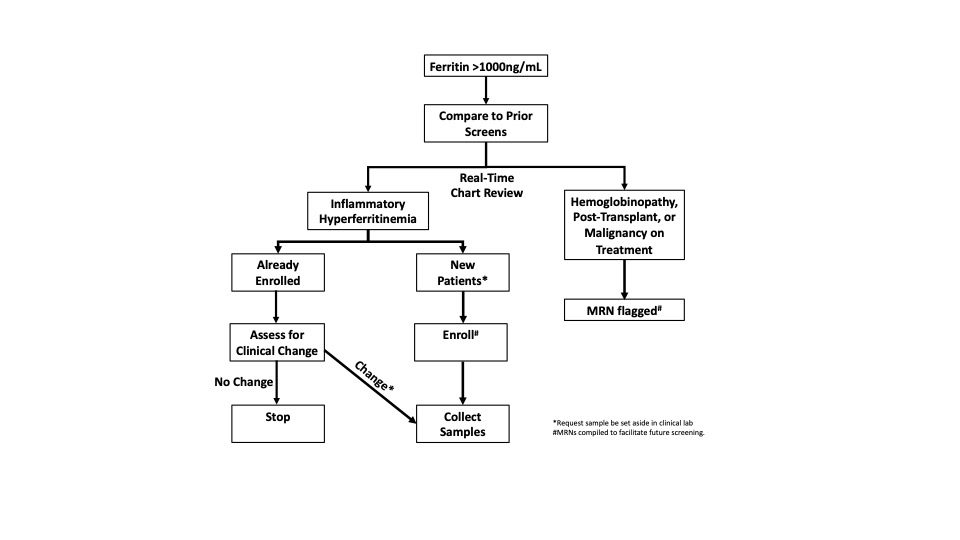
Methods: We instituted a single-center (UPMC Children’s Hospital of Pittsburgh) alert system from June 1st, 2017 to June 30th, 2019 wherein serum ferritin >1000ng/mL triggered via real-time chart review and attempted biobanking of remnant samples from willing patients deemed to have “inflammatory hyperferritnemia (IHF)” (Fig 1). From consenting patients we extracted relevant clinical data; retrospectively classified patients by etiology into infectious, rheumatic, or immune dysregulation; iteratively measured certain serum biomarkers (total IL-18, IL-18 binding protein, and CXCL9); and subjected a subgroup of samples to a 96-analyte O-link biomarker screen.
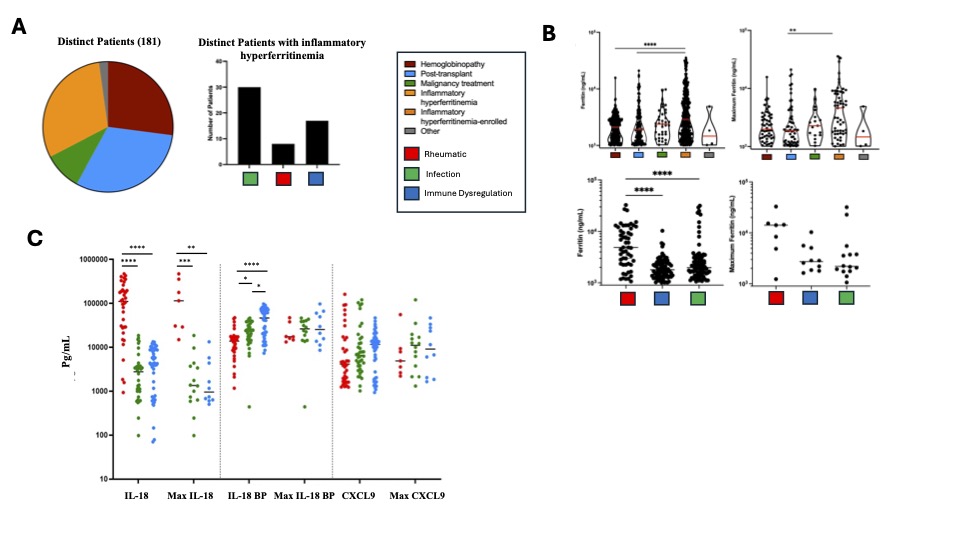
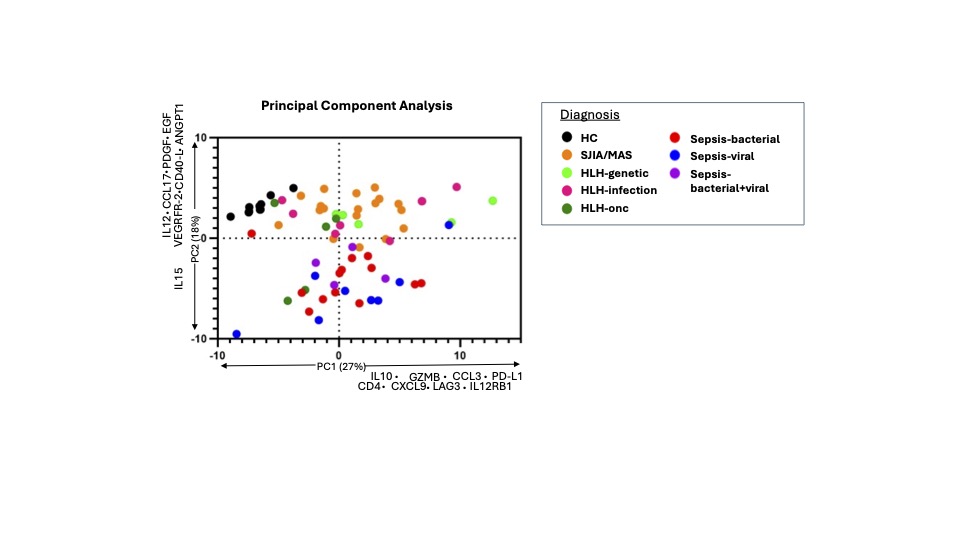
Results: The alert system identified 181 patients with hyperferritenemia, 30.5% of which had IHF (Fig 2A). Of the IHF patients, 14.5% were found to have a rheumatic cause, all of whom had systemic juvenile idiopathic arthritis (SJIA) (Fig 2A). Maximum ferritin levels were higher in IHF patients than either hemoglobinopathy or transplant but were not significantly different between subgroups of IHF (Fig 2B). Highly elevated total IL-18 levels were distinctive to SJIA with or without MAS compared to other IHF, whereas CXCL9 did not differentiate IHF subcategories (Fig 2C). Other lab values—triglycerides, AST, platelets, and fibrinogen—did not significantly differ between IHF subcategories. Principal component analysis of a 96-analyte biomarker screen showed distributed elevation of proteins associated with T-cell activation and IFNg-activity (e.g. Granzyme B, CXCL9) in all IHF samples. Samples from patients with hyperferritinemic sepsis were distinctively lower in proteins involved in vessel homeostasis (e.g. ANGPT1, VEGFR2) and antigen presentation (CD40L) compared to other IHF subgroups and healthy controls (Figure 3).
Conclusion: This single-center, real-time IHF screen proved feasible and efficient, validated prior observations about the specificity of IL-18, expanded our understanding of IHF heterogeneity, and enabled early sample collection from a unique and rapidly-evolving population.
To cite this abstract in AMA style:
Carol H, Mayer A, Varghese J, Martinez Z, Diorio C, Tsoukas P, Kernan K, Canna S. A Novel Hyperferritinemia Screen to Aid Differentiation of Hyperinflammatory Disorders [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/a-novel-hyperferritinemia-screen-to-aid-differentiation-of-hyperinflammatory-disorders/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-novel-hyperferritinemia-screen-to-aid-differentiation-of-hyperinflammatory-disorders/