Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) are subtypes of ANCA-associated vasculitis that frequently involve the kidneys. However, a subset of patients with GPA or MPA have no kidney involvement, and studies in this subgroup are limited. In the phase 3 ADVOCATE trial in patients with GPA or MPA, avacopan was noninferior to a prednisone taper in achieving remission at week 26 and superior in sustaining remission at week 52.1 This subgroup analysis focuses on efficacy, health-related quality of life (HRQoL), and safety outcomes of avacopan vs a prednisone taper in patients without active kidney involvement in the ADVOCATE trial.
Methods: ADVOCATE was a double-blind, double-dummy, active-controlled trial, with patients randomized 1:1 to receive avacopan (30 mg twice daily) or a prednisone taper (60 mg/day tapered to 0 by week 21) on a background of cyclophosphamide (followed by azathioprine or mycophenolate mofetil) or rituximab. Patients without kidney involvement at study baseline were identified from the Birmingham Vasculitis Score (BVAS) and key outcomes for this post hoc analysis were remission at week 26 and sustained remission at week 52. Other outcomes included glucocorticoid (GC) dose (prednisone-equivalent), Glucocorticoid Toxicity Index (GTI), HRQoL, and safety.
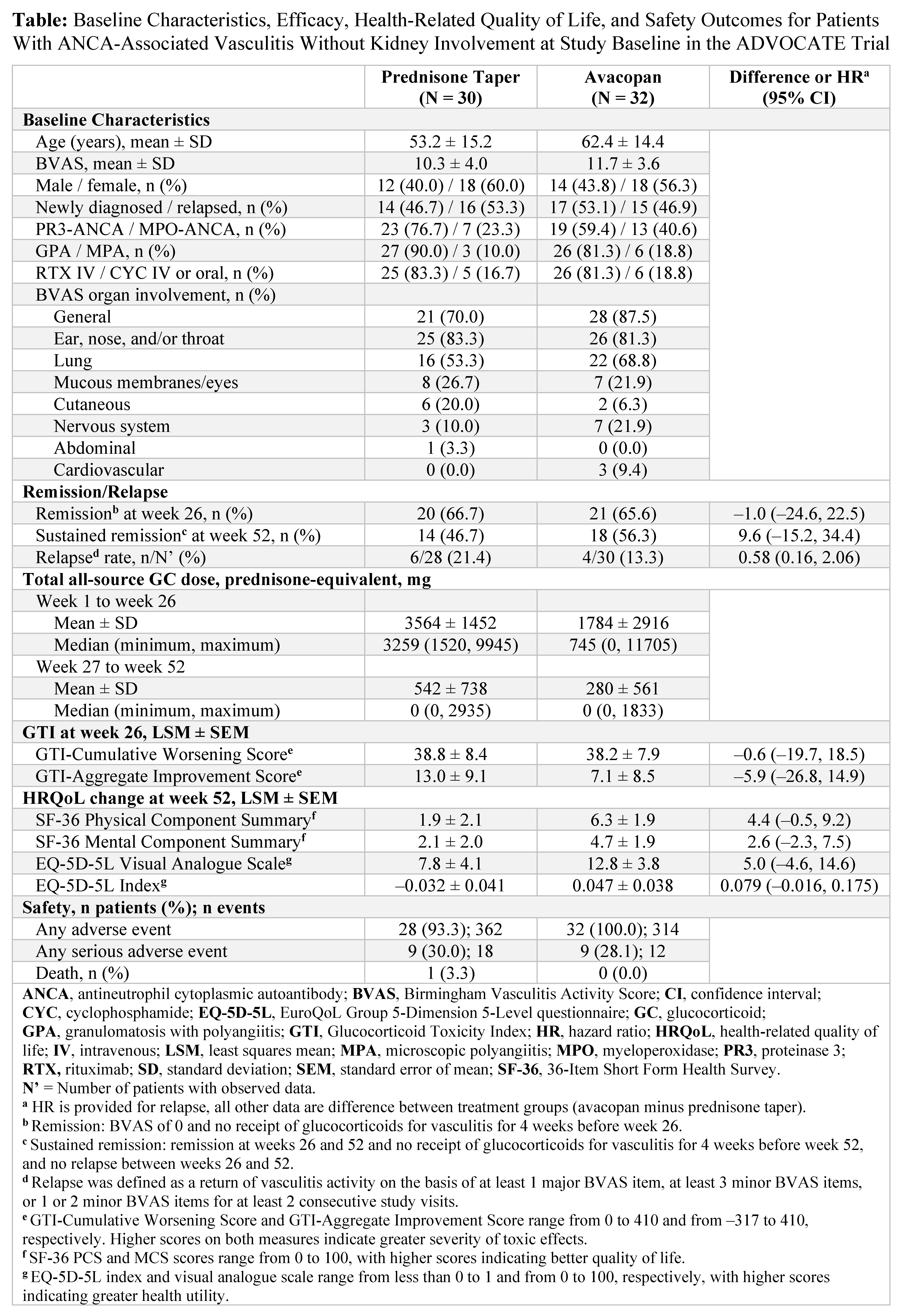
Results: Of the 331 patients enrolled in ADVOCATE, data from 62 (18.7%) patients (avacopan, N = 32; prednisone taper, N = 30) without kidney involvement at study baseline were analyzed. Most patients were female; had a diagnosis of GPA; had general, lung, ear, nose, and/or throat involvement; and received rituximab (Table). Mean ± SD baseline BVAS was 11.7 ± 3.6 with avacopan and 10.3 ± 4.0 with a prednisone taper. The mean/median total all-source GC dose was 1784/745 mg with avacopan vs 3564/3259 mg with a prednisone taper from week 1 to week 26 and 280/0 mg and 542/0 mg, respectively, from week 27 to week 52. A similar proportion of patients with avacopan vs a prednisone taper achieved remission at week 26 (65.6% vs 66.7%; difference: –1.0%; 95% CI: –24.6%, 22.5%) and a numerically higher proportion sustained remission at week 52 (56.3% vs 46.7%; difference: 9.6%; 95% CI: –15.2%, 34.4%). Relapse rate was numerically lower with avacopan vs a prednisone taper (13.3% vs 21.4%; HR: 0.58; 95% CI: 0.16, 2.06). GTI-Aggregate Improvement Score at week 26 was numerically lower (more favorable) with avacopan vs a prednisone taper (Table). The improvements from baseline in all HRQoL measures were numerically greater with avacopan vs a prednisone taper at week 52. Serious adverse events occurred in 9 patients (28.1%; 12 events) with avacopan and in 9 patients (30.0%; 18 events) with a prednisone taper; 1 death (3.3%) occurred (acute myocardial infarction) with a prednisone taper.
Conclusion: Findings from this post hoc subgroup analysis of ADVOCATE in patients with GPA or MPA without kidney involvement at study baseline demonstrate that avacopan was associated with a numerically higher rate of sustained remission, lower relapse rate, lower GC-related toxicity, greater improvements in HRQoL, and similar safety vs a prednisone taper.
Reference: 1. Jayne DRW, et al. N Engl J Med 2021;384(7):599-609.
To cite this abstract in AMA style:
Pagnoux C, Neel A, Bray S, Gurlin R, Trimpe D, Jayne D, Merkel P. Avacopan versus a Prednisone Taper in Patients with ANCA-Associated Vasculitis Without Kidney Involvement in a Phase 3 Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/avacopan-versus-a-prednisone-taper-in-patients-with-anca-associated-vasculitis-without-kidney-involvement-in-a-phase-3-trial/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/avacopan-versus-a-prednisone-taper-in-patients-with-anca-associated-vasculitis-without-kidney-involvement-in-a-phase-3-trial/