Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: The ACR Composite Response Index in diffuse cutaneous Systemic Sclerosis (CRISS) was developed to measure the probability of improvement in response to treatment in patients with early diffuse cutaneous SSc (dcSSc) [Khanna D et al. Arthritis Rheumatol 2016;68:299-311]. The revised CRISS calculates the proportion of patients who improve based on core measures, but does not discriminate non-improvement from worsening. With engagement from 180 patients and expert physicians, a continuous ranked composite important difference (RCID) score was developed to differentiate non-improvement from worsening, and to measure overall outcome, based on weighting minimal clinical important differences (MCIDs) in core CRISS domains [Bissell L et al. Arthritis Rheumatol 2022;74(suppl 9):0523]. We evaluated RCID scores in patients with dcSSc and interstitial lung disease (ILD) who participated in the SENSCIS trial.
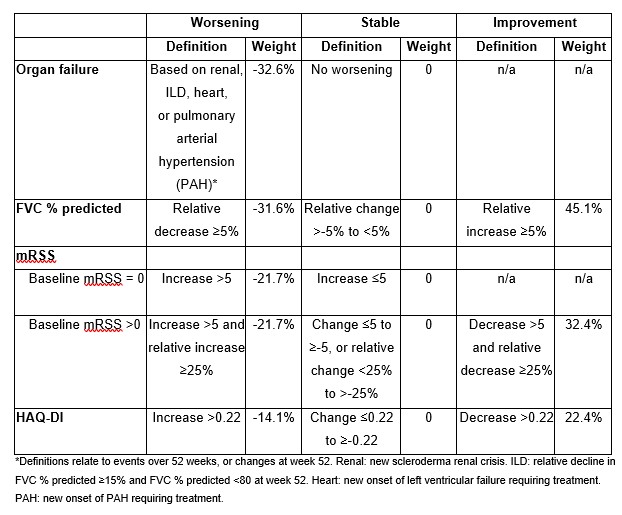
Methods: The SENSCIS trial enrolled patients with SSc-ILD with first non-Raynaud symptom in the prior ≤7 years and an extent of fibrotic ILD on HRCT ≥10%. Patients on prednisone ≤10 mg/day (or equivalent) and/or stable therapy with mycophenolate or methotrexate were allowed to participate. Patients were randomised to receive nintedanib or placebo. RCID scores over 52 weeks were calculated in the patients with dcSSc (overall and in subgroups by mycophenolate use). These scores were based on four components (organ failure, forced vital capacity [FVC] % predicted, modified Rodnan skin score [mRSS], Health Assessment Questionnaire Disability Index [HAQ-DI]) (Table) and ranged from -100% (worst outcome) to 100% (best outcome).
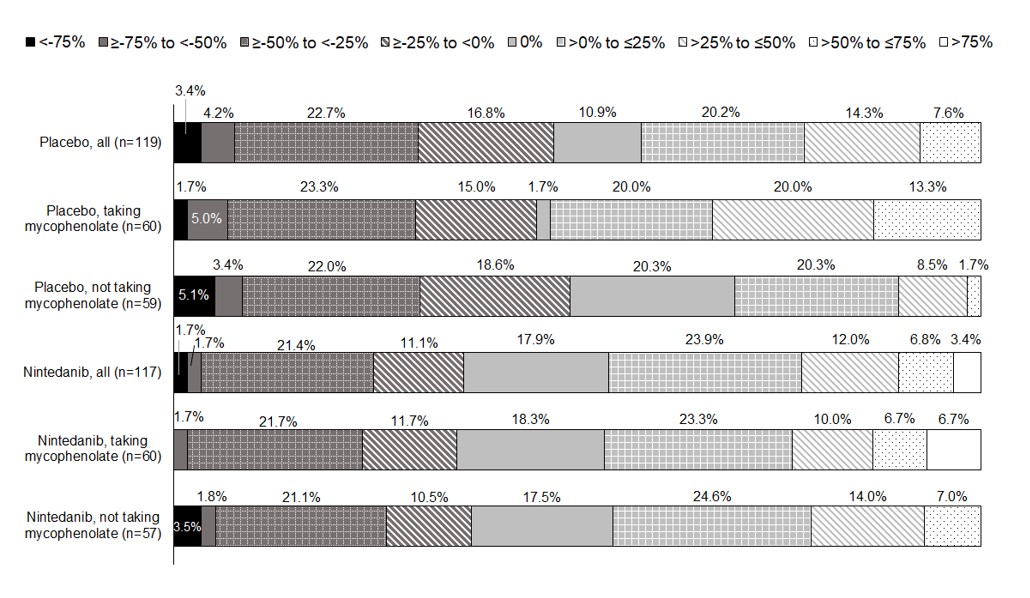
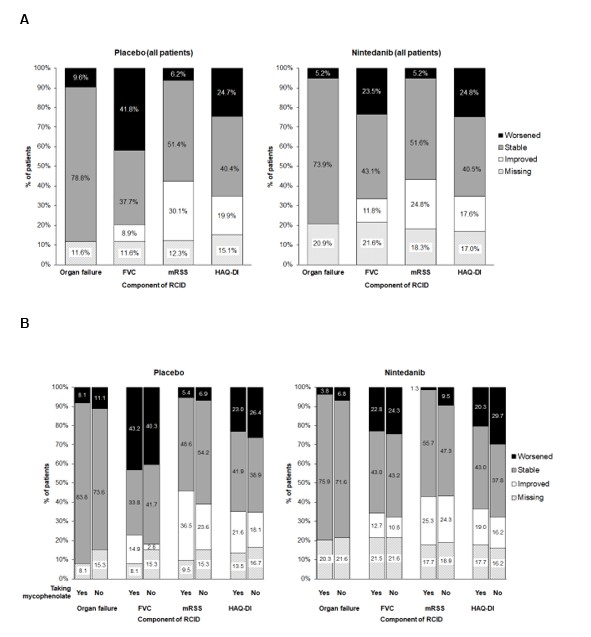
Results: In the placebo and nintedanib groups, respectively, mean (SD) RCID scores over 52 weeks were ‑3% (36) (n=119) and 4% (36) (n=117) overall, 3% (38) (n=60) and 7% (36) (n=60) among those taking mycophenolate at baseline, and ‑10% (31) (n=59) and < 1% (35) (n=57) among those not taking mycophenolate at baseline. Accordingly, a greater proportion of patients had stable or improved RCID scores (i.e. ≥0%) in the nintedanib group than the placebo group overall and in subgroups by use of mycophenolate (Figure 1). The proportions of patients with worsening of FVC % predicted was smaller in the nintedanib group than the placebo group overall (23.5% vs 41.8%) and in subgroups by use of mycophenolate (Figure 2). The proportions of patients with worsening/stability in other components of the RCID score were similar between the placebo and nintedanib groups (Figure 2).
Conclusion: Among patients with dcSSc and ILD in the SENSCIS trial, physician and patient-determined RCID scores were better overall in the nintedanib group than in the placebo group, mainly driven by reduced decline in FVC over 52 weeks.
To cite this abstract in AMA style:
Del Galdo F, Bissell L, Simonovska R, Alves M. Ranked Composite Important Difference (RCID) Scores in Patients with Diffuse Cutaneous Systemic Sclerosis and Interstitial Lung Disease [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/ranked-composite-important-difference-rcid-scores-in-patients-with-diffuse-cutaneous-systemic-sclerosis-and-interstitial-lung-disease/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/ranked-composite-important-difference-rcid-scores-in-patients-with-diffuse-cutaneous-systemic-sclerosis-and-interstitial-lung-disease/