Session Information
Date: Monday, November 18, 2024
Title: SLE – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Oral corticosteroids (OCS) are a mainstay in the clinical management of systemic lupus erythematosus (SLE). However, prolonged use may result in systemic side effects.1 Thus, identifying therapies that effectively manage SLE while reducing OCS is vital. Clinical studies on first-in-class type I interferon inhibitor anifrolumab have shown a reduction in OCS dose and flares among SLE patients.2,3 In this study, we assess real-world OCS use before and during anifrolumab therapy among adults with SLE in the United States (US).
Methods: This retrospective, observational cohort study included data from patients with SLE in the American Rheumatology Network. Patients included were ≥18 years initiating anifrolumab (index date) from August 2, 2021 and followed until December 31, 2023, with ≥180 days of clinical history pre-index and ≥360 days of follow-up post-index, serum creatinine ≤2.0 mg/dL and urine protein-creatinine ratio ≤2.0 mg/mg. Participants received OCS at baseline and adhered to anifrolumab during follow-up (≥6 infusions from index to day 360 post-index). Outcomes assessed included: 1) OCS use (mean daily OCS dose [prednisone equivalents, PE] for days supplied, proportion of patients receiving OCS, and with mean daily OCS dose >5 mg or >7.5 mg); 2) clinical disease assessments (SLEDAI-2K, complement C3/C4). Outcomes were assessed during 180-day (6-month) periods at baseline (day -180 to -1 pre-index), and during follow-up (1-180 days post-index [Period 1], and 181-360 days post-index [Period 2]). The result closest to the end of each 180-day period was chosen per patient, with group estimates reported.
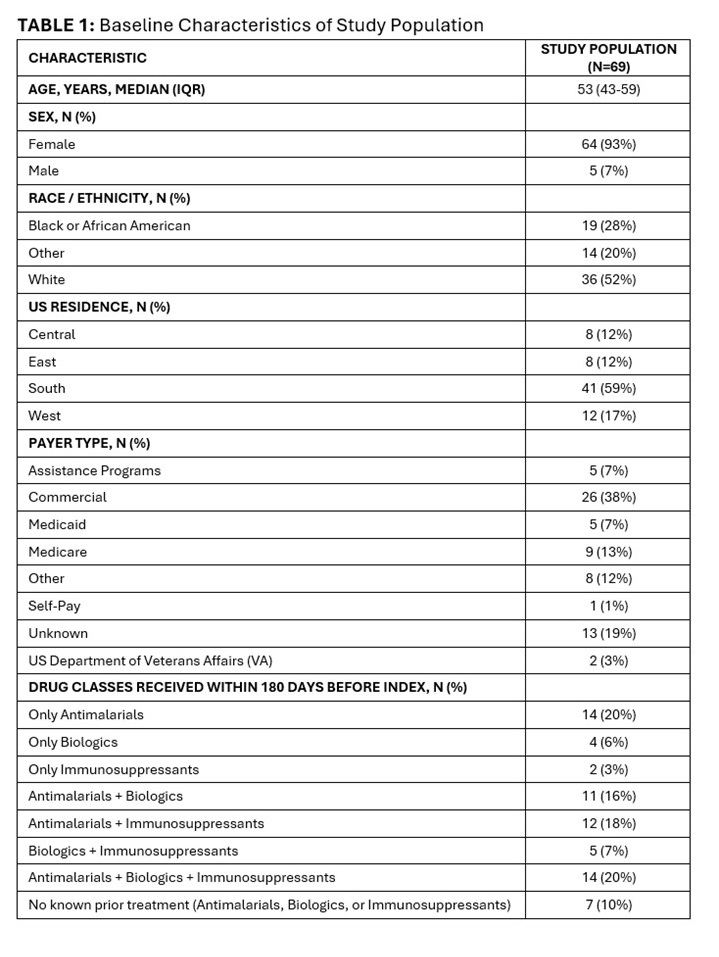
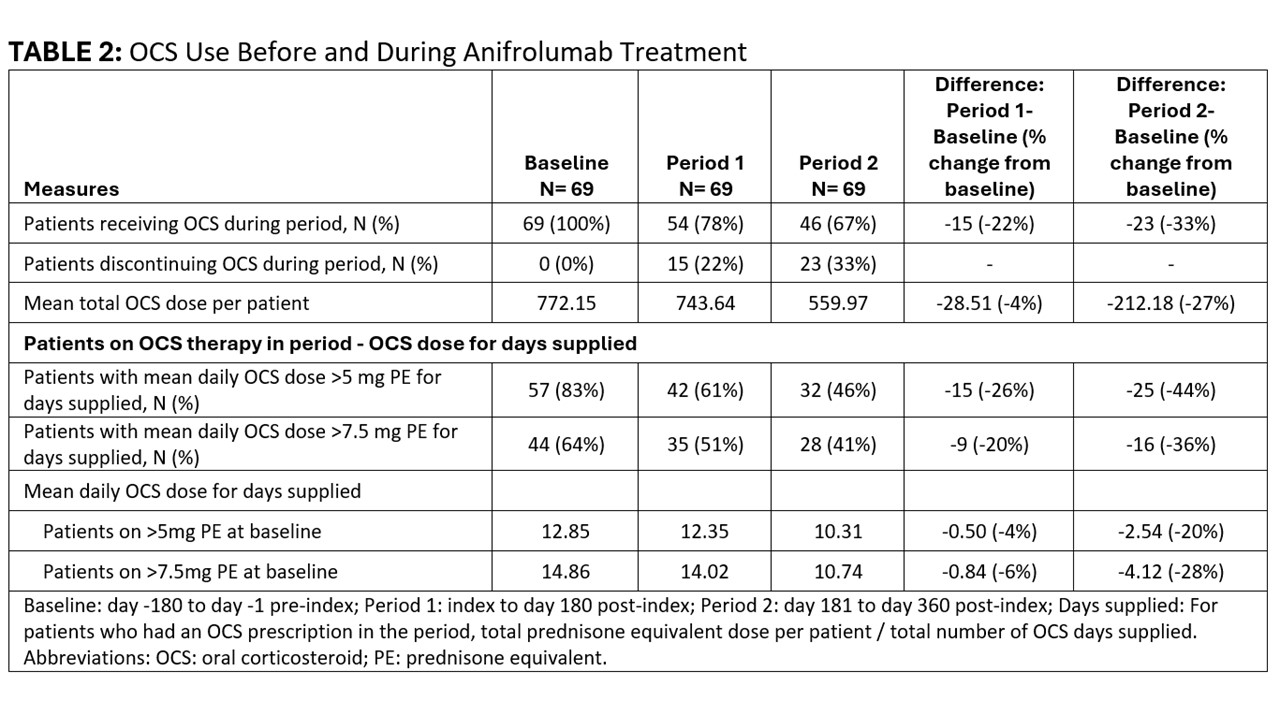
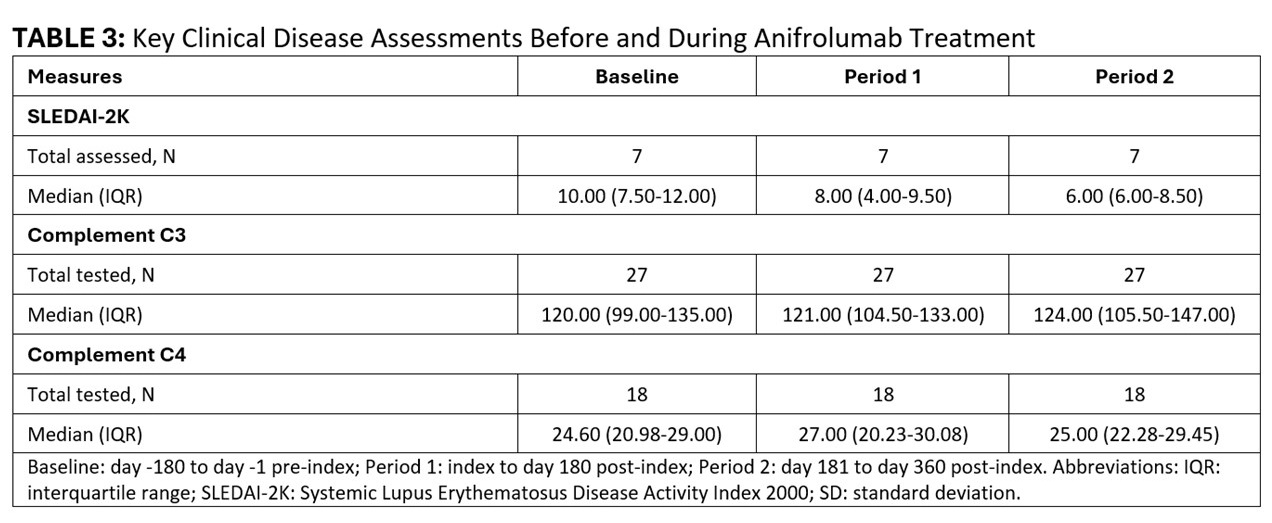
Results: The 69 participants on OCS therapy at baseline were mostly female (93%), white (52%), with median age 53 (IQR 43-59) years. [TABLE 1] An increasing number of patients discontinued OCS during follow-up (Period 1: n=15, 22%; Period 2: n=23, 33%). [TABLE 2] The mean total OCS dose per patient decreased from 772.15 mg at baseline to 743.64 (-4%) in Period 1 and 559.97 (-27%) in Period 2. Over time, fewer patients had mean daily OCS dose >5 mg (baseline: 57; Period 1: 42; Period 2: 32), and >7.5 mg (baseline: 44; Period 1: 35; Period 2: 28), with ≥20% reduction in mean daily dose for these high-dose OCS groups by Period 2 ( >5mg group: -20%; >7.5 mg group: -28%). [TABLE 2] Where available, median SLEDAI-2K scores also declined during follow-up (baseline: 10; Period 1: 8; Period 2: 6). [TABLE 3]
Conclusion: In this real-world cohort of patients with SLE on anifrolumab therapy, a reduction in OCS use was noted within the first six months of anifrolumab initiation. Treatment benefits increased with longer duration of anifrolumab therapy, with large reductions in the number of patients receiving high-dose OCS, OCS dose reduction among patients on high-dose OCS, and a third of patients discontinuing OCS by the end of year one. Decreased OCS use was accompanied by a decline in disease activity, as assessed with SLEDAI-2K. These findings provide real-world evidence of OCS sparing with anifrolumab therapy together with reduction in disease activity.
References:
1. Frodlund M, et al. Rheumatology. 2024;63(4):1104-1112
2. Furie R, et al. Lupus. 2021;30(8):1254-1263
3. Kalunian K, et al. Arthritis Rheumatol. 2023;75(2):253-265
To cite this abstract in AMA style:
Kyttaris V, Atefi G, Persons D, Withrow D, Binka M. Reduction in Oral Corticosteroid Use During Anifrolumab Therapy: Observations from a Real-world Cohort of Adults with Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/reduction-in-oral-corticosteroid-use-during-anifrolumab-therapy-observations-from-a-real-world-cohort-of-adults-with-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/reduction-in-oral-corticosteroid-use-during-anifrolumab-therapy-observations-from-a-real-world-cohort-of-adults-with-systemic-lupus-erythematosus/