Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: A rare subset of children who contract SARS-CoV-2 later develop the hyperinflammatory condition known as multisystem inflammatory syndrome in children (MIS-C). There has been hesitation to vaccinate these children against COVID-19 due to concerns for a recurrence of hyperinflammation. As part of post-MIS-C follow-up care, we have advocated for COVID-19 vaccination. We aimed to prospectively evaluate reactogenicity and safety following COVID-19 mRNA vaccination among patients with a history of MIS-C compared to healthy children (HC) without a history of inflammatory disease.
Methods: From February 2023 to February 2024, patients 6 months to 20 years of age with a history of MIS-C or HC were enrolled in an IRB-approved study and monitored for 6 months following COVID-19 mRNA vaccination, administered according to age-specific recommendations (standard of care). Prospective reactogenicity data and accompanying impact on quality of life (severity) were collected by survey daily for 7 days following receipt of each vaccine. Pairwise comparisons were performed using Wilcoxon rank-sum for continuous variables and Fisher’s exact test for categorical variables.
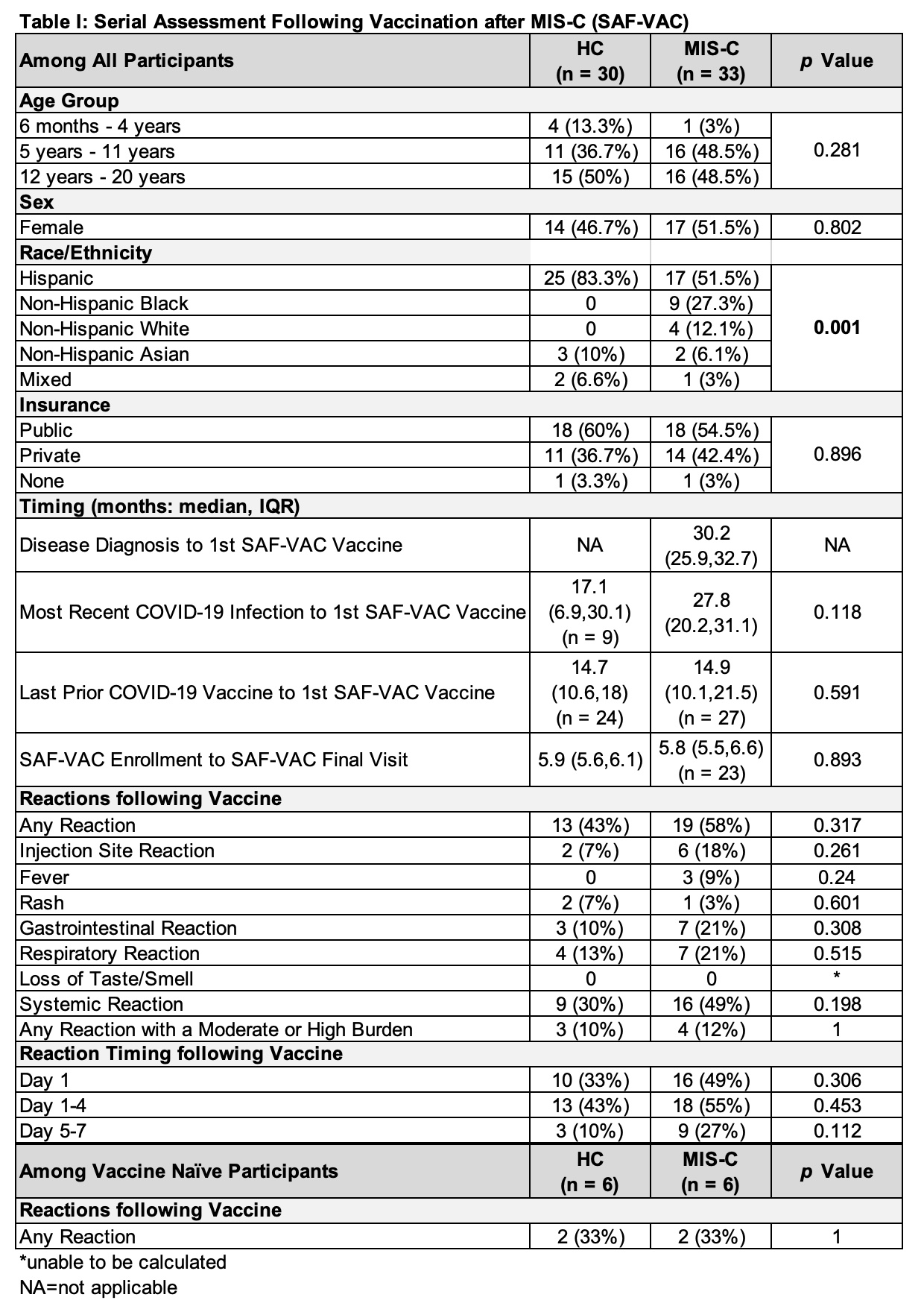
Results: We enrolled 33 patients with a history of MIS-C and 30 HC in the Serial Assessment Following Vaccination After MIS-C (SAF-VAC) study. Patients with MIS-C were diagnosed a median of 30.2 months prior to SAF-VAC vaccination [IQR: 25.9, 32.7]. There were no differences in age, sex, or insurance coverage between the two groups; however, fewer HC were Non-Hispanic Black and White and more were Hispanic compared to patients with a history of MIS-C (p< 0.001). 9 of 30 (30%) HC reported a documented previous COVID-19 infection a median of 17.1 months [IQR 6.9, 30.1] prior to SAF-VAC vaccination. Median time between last known COVID-19 infection and SAF-VAC vaccination in patients with a history of MIS-C was 27.8 months [IQR: 20.2, 31.1]. In total, 427 of 441 (97%) reactogenicity surveys from 33 patients with a history of MIS-C and 30 HC were returned. There were no differences in self-reported reactogenicity or severity following COVID-19 vaccination, or in timing of any reported reaction between the two groups (Table I). Most enrolled individuals had been previously vaccinated: 27 (82%) patients with a history of MIS-C 14.9 [IQR: 10.1, 21.5] months prior to SAF-VAC vaccination, and 24 (80%) HC 14.7 [IQR: 10.6, 18] months prior to SAF-VAC vaccination (p=0.591). Among the 6 vaccine naïve individuals in each group, there were no differences in reactogenicity (p=1). There were no concerns for flares of hyperinflammation among patients with a history of MIS-C during 5.8 [IQR: 5.5, 6.6] months of monitored followup.
Conclusion: This cohort of prospectively followed patients with a history of MIS-C tolerated COVID-19 mRNA vaccination with no increase in reactogenicity compared to HC, and without evidence of inflammatory flares. Our results suggest that patients with a history of MIS-C can safely receive COVID-19 mRNA vaccination. These results are especially reassuring as SARS-CoV-2 becomes endemic and annual vaccination against SARS-CoV-2 is recommended.
To cite this abstract in AMA style:
Sanchez Villa M, Nguyen J, Guffey D, Sahni L, Gillispie-Taylor M, De Guzman M, Sexson Tejtel S, Devaraj S, Munoz F, Vogel T. Prospective Evaluation of Reactogenicity and Safety Following COVID-19 Vaccination in Children with a History of MIS-C [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/prospective-evaluation-of-reactogenicity-and-safety-following-covid-19-vaccination-in-children-with-a-history-of-mis-c/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/prospective-evaluation-of-reactogenicity-and-safety-following-covid-19-vaccination-in-children-with-a-history-of-mis-c/