Session Information
Date: Monday, November 18, 2024
Title: Osteoporosis & Metabolic Bone Disease – Basic & Clinical Science Poster
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Leucocyte cell derived chemotaxin 2 (LECT2) is a hepatokine produced in liver cells, secreted into the circulation, and acts as a hormone throughout the body. It is involved in the pathogenesis of several diseases such as liver disease, sepsis, degenerative arthritis, and rheumatoid arthritis. In addition, it is reported to be identical to chondromodulin-II, a cartilage regulator that stimulates and activates the proliferation of chondrocytes and osteoblasts. However, research on the role and mechanism of action of LECT2 on osteoblasts and osteoclasts that regulate bone metabolism has not been revealed.Therefore, in this study, we attempted to determine the cause of osteoporosis due to imbalance in bone metabolism in vivo by revealing the effect and mechanism of action of the LECT2 gene on bone metabolism.
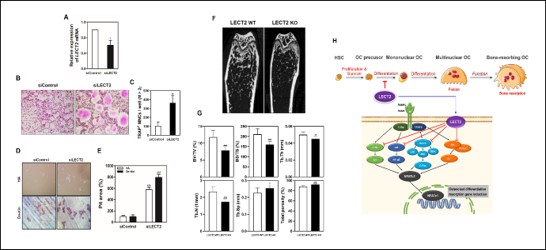
Methods: The osteoclast differentiation effect of LECT2 was each evaluated by TRAP staining and dentin bone resorption assay using LECT2 recombinant protein and siRNA silencing, and the intracellular signaling pathway was examined through real-time quantitative RT-PCR and western blot analysis. Also, LECT2 gene-deficient mice were created to demonstrate the effect of LECT2 on bone metabolism in vivo, and osteological characteristics of LECT2 gene-deficient mice were confirmed through micro-CT and histological analysis.
Results: We found that LECT2 positively regulated the receptor activator of nuclear factor kappa B ligand (RANKL)-induced osteoclast differentiation and bone resorption with no effects on osteoblast differentiation by performing gain- and loss-of-function studies. Based on the in vitro experimental results, an experiment was conducted comparing LECT2 gene-deficient mice with wild-type mice. It was confirmed through micro-CT and histological analysis that the bone loss phenomenon in mice deficient in the LECT2 gene was further increased. In addition, it was confirmed through TRAP staining and F-actin analysis that osteoclast differentiation in bone marrow cells of LECT2 gene-deficient mice further progressed by RANKL. Additionally, in LECT2 gene-deficient mice, phosphorylation of Akt, p38, JNK, ERK, IκB and PLCγ2 and mRNA expression of OC-STAMP, DC-STAMP, β3-integrin, MMP9, Atp6v0d2, Cathepsin K and OSCAR were significantly increased, and expression of c-Fos and NFATc1 was increased at both protein and mRNA levels.
Conclusion: As a result, the significance of the in vitro and in vivo results demonstrated that the LECT2 gene plays an important role in bone metabolism by regulating osteoclast differentiation and function, and that LECT2 has potential as a candidate for gene therapy related to bone diseases.
To cite this abstract in AMA style:
Lee M, Lee C, CHUNG C, Kim J, Cheon Y, Eun S, Park G. Role of LECT2 in the Regulation of Bone Metabolism In Vitro and In Vivo [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/role-of-lect2-in-the-regulation-of-bone-metabolism-in-vitro-and-in-vivo/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/role-of-lect2-in-the-regulation-of-bone-metabolism-in-vitro-and-in-vivo/