Session Information
Date: Monday, November 18, 2024
Title: Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Nephrolithiasis and gout are both common, extremely painful conditions which frequently coexist, along with type 2 diabetes (T2D). Sodium-glucose cotransporter-2 inhibitors (SGLT2i), first approved for T2D, increase urinary flow with osmotic diuresis and uric acid excretion, both which could impact nephrolithiasis risk. However, data on SGLT2i and nephrolithiasis recurrence are limited, and not available among gout patients.
Our objective was to compare risk of recurrent nephrolithiasis and gout flares among gout patients with T2D initiating SGLT2i versus glucagon-like peptide-1 receptor agonists (GLP1RA) or dipeptidyl peptidase 4 inhibitors (DPP4i), all contemporary second-line glucose lowering agents.
Methods: We designed and conducted target trial emulations (TTEs) to compare SGLT2i vs GLP1RA or DPP4i for risk of recurrent nephrolithiasis among adults with T2D and preexisting nephrolithiasis overall (TTE#1), and among the subset also with preexisting gout (TTE#2). We used a Canadian general population database from Jan 2014 to June 2022, including all dispensed prescriptions. Randomization was emulated using inverse probability of treatment weights (primary analysis) and overlap weighting (sensitivity analysis) based on > 50 preexposure variables including duration and severity of gout, nephrolithiasis, and T2D.
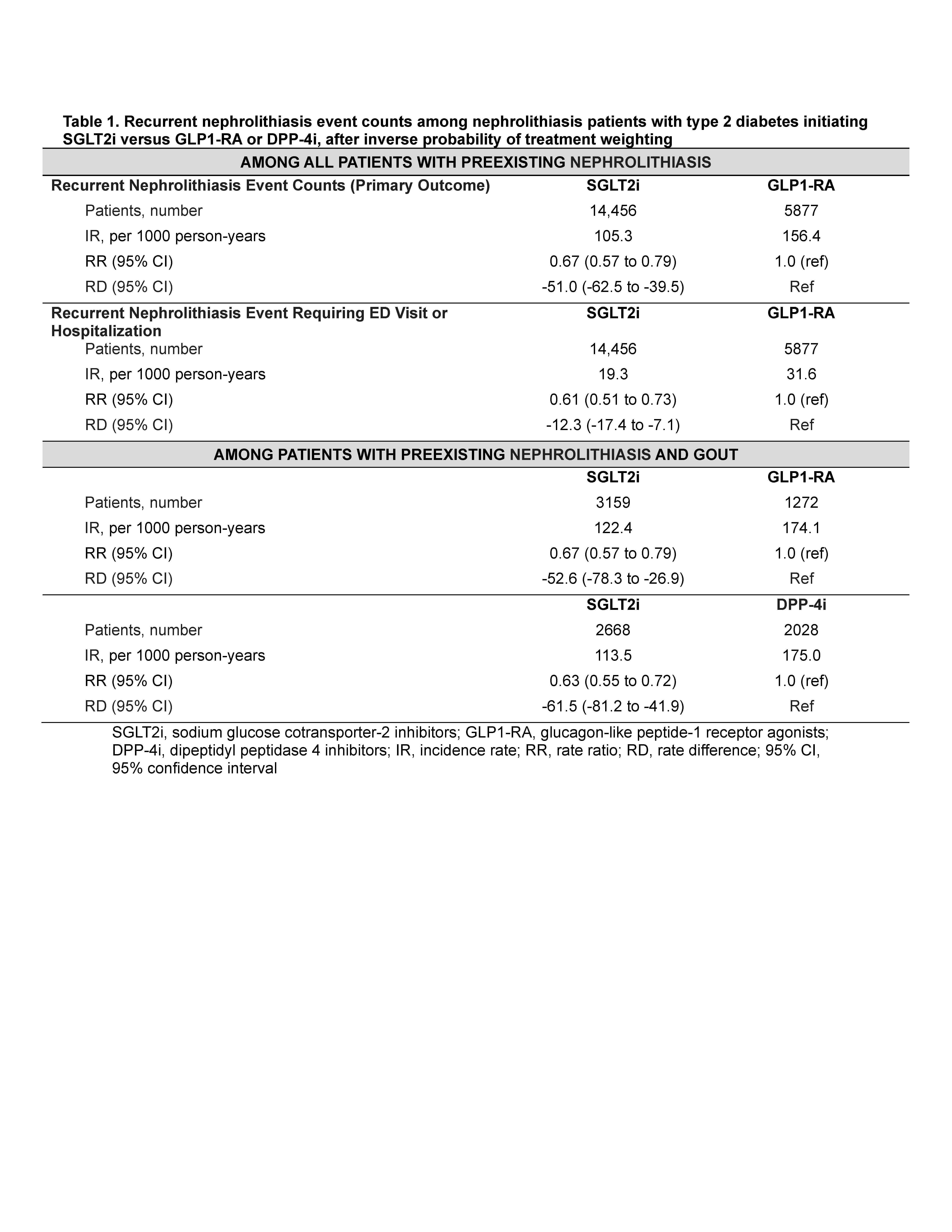
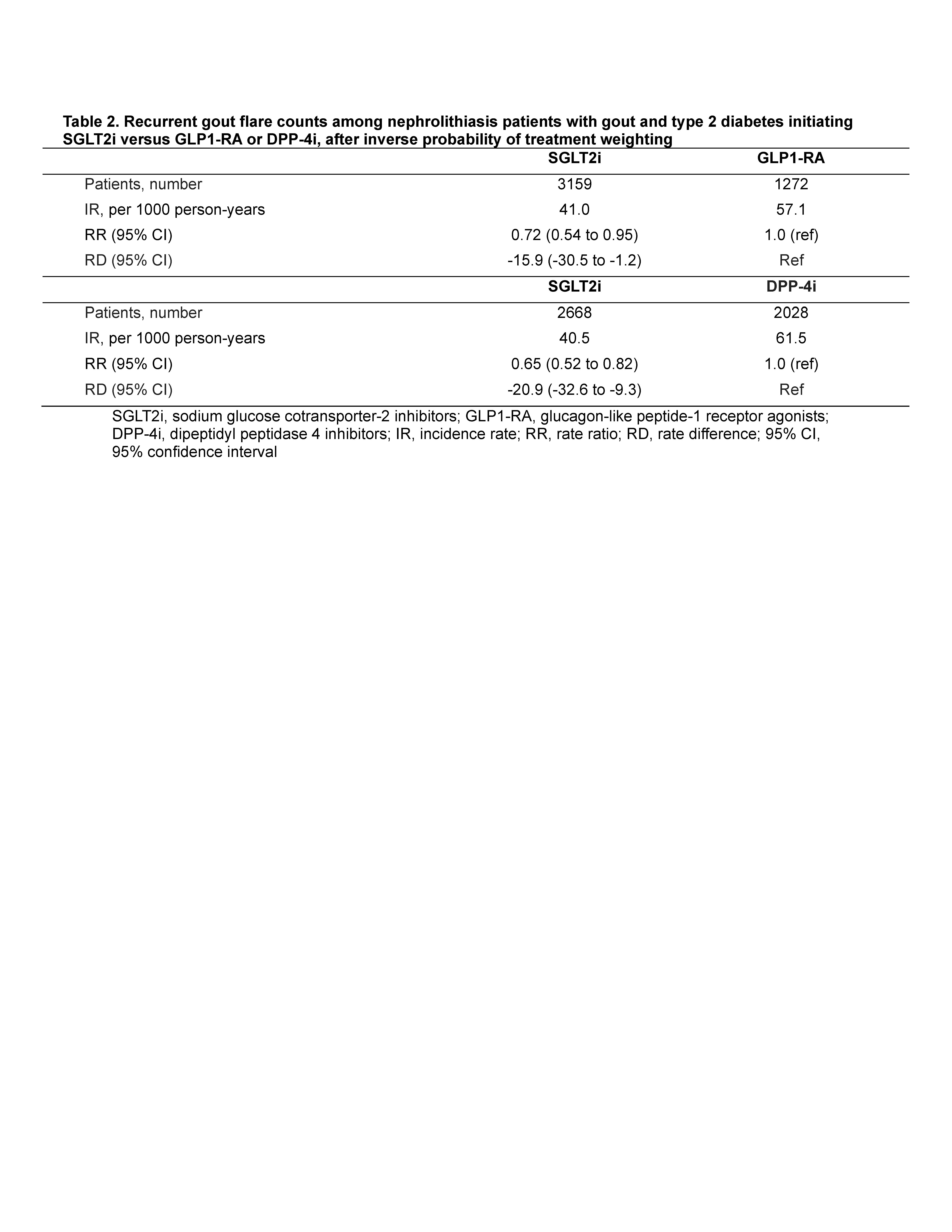
Nephrolithiasis episodes were ascertained by emergency department (ED), hospitalisation, or outpatient visits (PPV ≥90% [Semins et al. 2010]). Secondary outcomes included nephrolithiasis resulting in hospitalization/ED visits and requiring procedures. We also assessed rate of recurrent gout flares, ascertained from ED, hospitalization, outpatient, and medication dispensing records (PPV 95% for≥ 1 flare over a similar period).
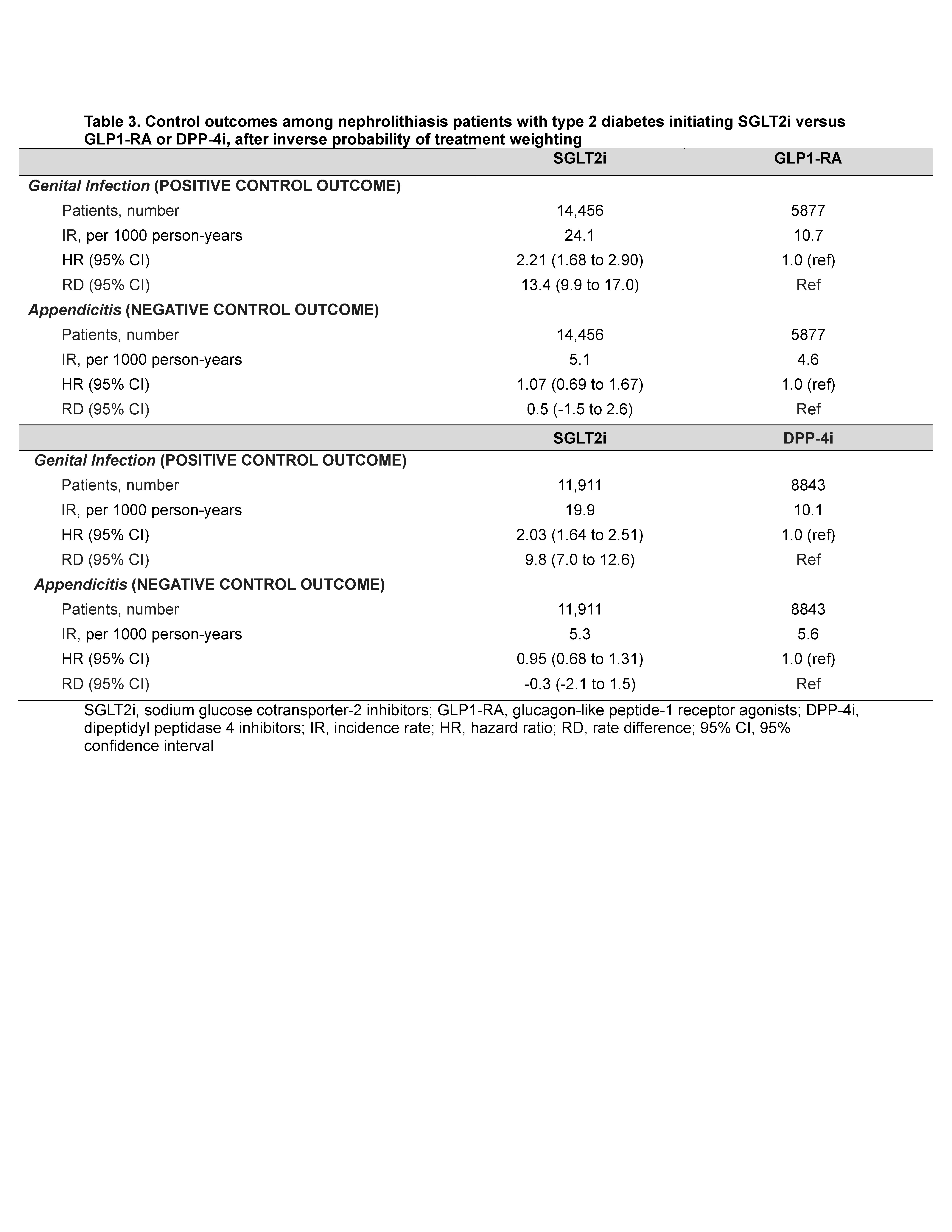
We included genital infection as positive control and appendicitis as negative control outcomes. Weighted Poisson and Cox proportional hazard models were used to calculate rate ratios (RR), hazard ratios, and rate differences (RD).
Results: Baseline characteristics were well-balanced after inverse probability weighting (all SMD < 0.1). Rates of recurrent nephrolithiasis were lower among those initiating SGLT2i vs GLP1RA or DPP4i (Table 1). Comparing SGLT2i vs GLP1RA among patients with preexisting gout, RR was 0.67 (95% CI: 0.57, 0.79) and RD was -53 events (-78, -27) per 1000 person-years [PY]).
SGLT2i initiation was also associated with reduced rate of recurrent gout flares, with RR 0.72 (0.54, 0.95) and RD -16 flares [-31, -1.2] per 1000 PY) (Table 2).
Findings were consistent using DPP4i as the active comparator (Tables 1-2) and using overlap weighting. Protective associations also persisted for nephrolithiasis requiring ED visits/hospitalizations or procedures (Table 1), while SGLT2i initiators had higher risk of genital infection, and no altered risk of appendicitis, as expected (Table 3).
Conclusion: The dual added benefits of SGLT2i for gout patients beyond established diabetes therapy (i.e., reduced rate of recurrent nephrolithiasis and gout flares) suggest this class of medication may be an ideal multi-purpose addition to current gout or nephrolithiasis therapies to address the high burden of flares, stone, and comorbidities.
To cite this abstract in AMA style:
McCormick N, Yokose C, Lu L, Wexler D, Avina-Zubieta J, De Vera M, chigurupati s, Tan K, Chen C, McCoy R, Curhan G, Choi H. The Dual Benefits of Sodium-Glucose Cotransporter-2 Inhibitors for Recurrent Nephrolithiasis and Gout Flares Among Gout Patients with Type 2 Diabetes: New User, Active Comparator Target Trial Emulation Studies [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/the-dual-benefits-of-sodium-glucose-cotransporter-2-inhibitors-for-recurrent-nephrolithiasis-and-gout-flares-among-gout-patients-with-type-2-diabetes-new-user-active-comparator-target-trial-emulatio/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-dual-benefits-of-sodium-glucose-cotransporter-2-inhibitors-for-recurrent-nephrolithiasis-and-gout-flares-among-gout-patients-with-type-2-diabetes-new-user-active-comparator-target-trial-emulatio/