Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Immune checkpoint inhibitors (ICIs) produce immune-related adverse events in patients with rheumatic diseases that can often present as a flare of the underlying condition. Efforts to understand these events have primarily focused on patients with traditionally recognized autoimmune diseases such as rheumatoid arthritis, but have not addressed the impact of ICIs in autoinflammatory diseases such as gout. Gout flares have been reported following ICI treatment, but there are no population-based investigations of the impact of ICI treatment in gout. This study assessed the incidence, features, and potential risk factors associated with gout flares following ICI treatment in Veterans receiving care in rheumatology clinics in the Veterans Health Administration (VHA).
Methods: US Veterans enrolled in VHA who had received an ICI and had a rheumatology clinic visit within 6 months after first ICI infusion were identified. Electronic medical record (EMR) review classified patients as having established gout if the diagnosis was documented prior to ICI infusion by a rheumatology provider. For each patient with an established gout diagnosis, EMR review during the six months after ICI infusion applied the following criteria to identify gout flares: 1) patient or provider report of a flare and 2) at least one associated symptom/sign of warmth, swelling, and/or pain; and/or treatment of the flare with corticosteroids, NSAIDS, or colchicine. Features of gout recorded included history of previous flares, past use of urate lowering therapy (ULT), tophi, and documentation of monosodium urate (MSU) crystals were recorded. Data from the VA Corporate Data Warehouse (CDW) was extracted to provide basic demographic and laboratory data.
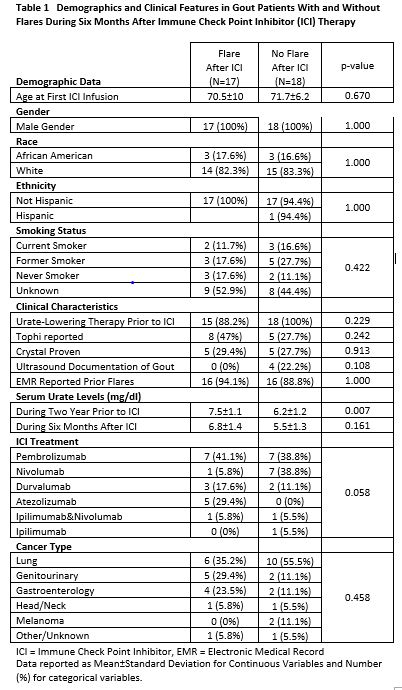
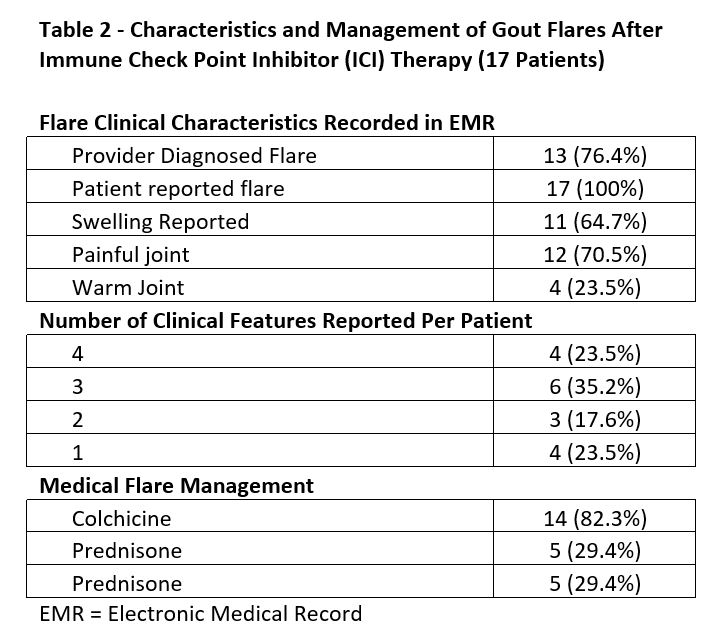
Results: Of the 34,344 patients receiving ICI therapy in VHA, 308 had rheumatology appointments within 6 months after ICI infusion. Of these 308 patients, 35 (11.3%) had an established prior diagnosis of gout. Of the 35 gout patients, 17 (48.5%) met criteria for an acute gout flare within six months of ICI treatment. These patients were 100% male, predominantly white, and with a mean age at ICI initiation of 71.1 years (Table 1). The clinical features and treatment of flares are reported in Table 2. Treatment of flares with medication was reported in 14 of the 17 patients, colchicine being the most common drug chosen. The small sample size limits the interpretation of potential risk factors. A potential signal for flares was seen in patients with higher serum urate levels prior to ICI, taking atezolizumab, and those with GI and GU cancers. However, these observations require further investigation to fully understand any potential associations.
Conclusion: This nationwide evaluation of VHA patients with established gout identified gout flares in 48.5% of patients during the six months following ICI infusion. While flares may certainly occur in patients with gout as a part of the natural history of this disease, the high frequency of flares after ICI therapy warrants additional investigation. Larger studies with control populations not treated with ICIs are needed to further understand the increased flare risk following ICI treatment in gout patients.
To cite this abstract in AMA style:
herron A, Ting Lai M, Schlesinger N, brian S, Rojas J, Patel s, O’Sullivan M, Cannon g, Braaten T. Gout FlaresFollowingImmune Checkpoint Inhibitors Treatment [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/gout-flaresfollowingimmune-checkpoint-inhibitors-treatment/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/gout-flaresfollowingimmune-checkpoint-inhibitors-treatment/