Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Several peripheral biomarkers for RA-associated interstitial lung disease (RA-ILD) have been evaluated to enhance early RA-ILD identification. The MUC5B rs35705950 promoter variant, matrix metalloproteinase-7 (MMP-7), and anti-malondialdehyde-acetaldehyde (MAA) antibodies are among the most promising peripheral biomarkers for RA-ILD identification discovered to date. We investigated whether a score based on elevations in these three biomarkers would stratify prevalent and incident RA-ILD risk.
Methods: We performed cross-sectional and cohort studies within a multicenter, prospective cohort of U.S. veterans with RA. RA-ILD was systematically identified in the cohort, and all cases were validated based on standardized medical record review by a board-certified rheumatologist requiring a clinical diagnosis and supportive imaging or biopsy findings. MUC5B promoter variant was measured using the Infinium Global Screening Array, and autosomal dominant inheritance was assumed. MMP-7 was measured from plasma using the MesoScale Discovery platform. IgM anti-MAA-albumin antibodies were measured by ELISA in the serum. A combined three biomarker score (range 0-3) was calculated from the presence of the MUC5B variant and elevations (upper 25% vs. lower 75%) in MMP-7 and anti-MAA antibody concentrations. Multivariable logistic (prevalent RA-ILD) and Cox (incident RA-ILD) regression models evaluated score performance adjusting for age, sex, race, smoking status, BMI category, anti-CCP antibody positivity, DAS28, and Rheumatic Disease Comorbidity Index Score.
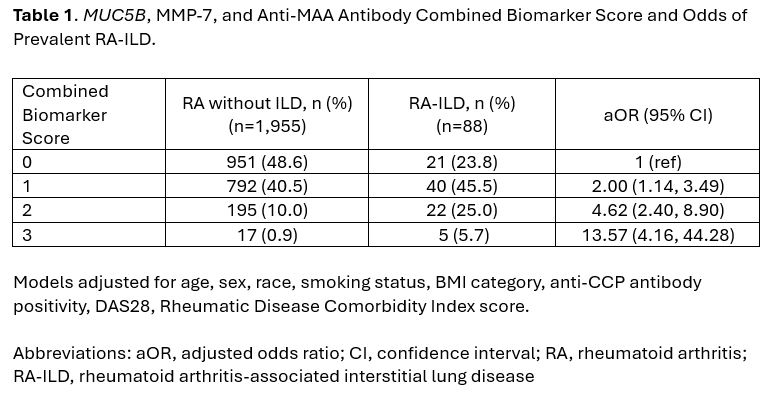
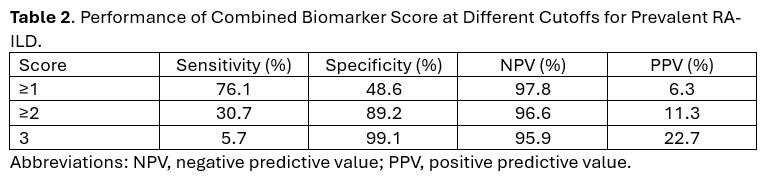
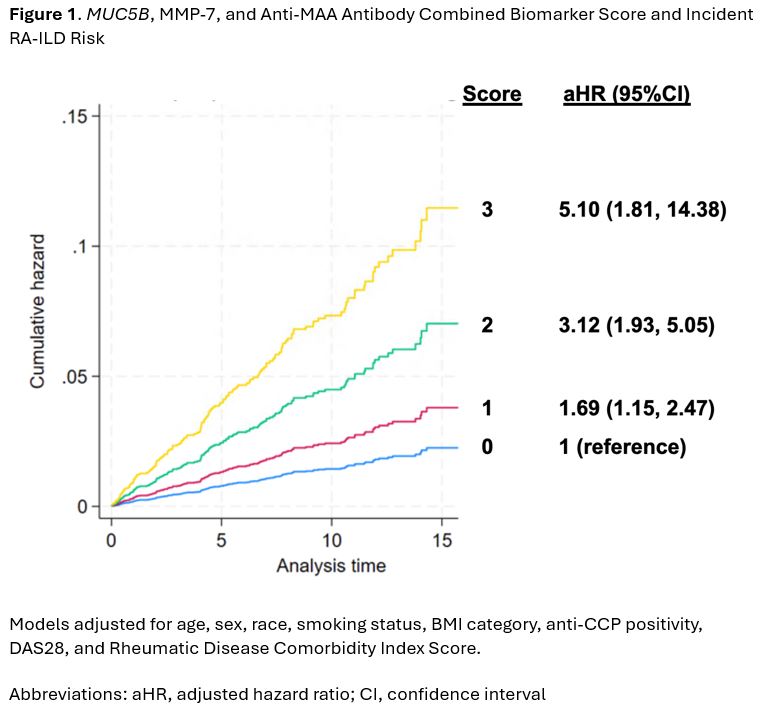
Results: We studied 2,231 RA participants (89% male, mean age 64 years), with 88 having prevalent RA-ILD and 148 developing incident RA-ILD over 16,905 patient-years of follow-up. Among those with prevalent RA-ILD, 76% had at least one elevated biomarker and 31% had at least two elevated biomarkers (i.e., sensitivity). Referent to those with no elevated biomarkers (2.2% RA-ILD prevalence), RA patients with 1 (4.8% RA-ILD prevalence, aOR 2.00), 2 (10.1% RA-ILD prevalence, aOR 4.62), or 3 (22.7% RA-ILD prevalence, aOR 13.57) elevated biomarkers had significantly higher odds of prevalent RA-ILD (Table 1). A score of ≥2 had 89% specificity for prevalent RA-ILD (Table 2). Negative predictive values were high and positive predictive values were low, reflecting the low prevalence of RA-ILD. Among those who developed incident RA-ILD, 67.6% had at least one elevated biomarker and 23.0% had at least two elevated biomarkers. In incident RA-ILD analyses (Figure 1), RA patients with combined biomarker scores of 1 (aHR 1.69), 2 (aHR 3.12), or 3 (aHR 5.10) had a significantly increased risk of developing incident RA-ILD during follow-up compared to those with no elevated biomarkers.
Conclusion: A combined biomarker score based on MUC5B, MMP-7, and anti-MAA antibody risk stratified RA patients for prevalent and incident RA-ILD. Because these biomarkers have been previously studied within this cohort, external validation of the biomarker score is needed to further evaluate if this score could be utilized to improve RA-ILD screening approaches.
To cite this abstract in AMA style:
England B, Wheeler A, Luedders B, Duryee M, Frideres H, Wysham K, Cannon g, Kunkel G, Ascherman D, Monach P, Kerr G, Reimold A, Baker J, Thiele G, Mikuls T. Combining Three Peripheral Biomarkers to Stratify Rheumatoid Arthritis-Associated Interstitial Lung Disease Risk [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/combining-three-peripheral-biomarkers-to-stratify-rheumatoid-arthritis-associated-interstitial-lung-disease-risk/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/combining-three-peripheral-biomarkers-to-stratify-rheumatoid-arthritis-associated-interstitial-lung-disease-risk/