Session Information
Date: Sunday, November 17, 2024
Title: Abstracts: RA – Diagnosis, Manifestations, & Outcomes III: Best Day (RA Subpopulations)
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Current treatment guidelines for rheumatoid arthritis (RA) recommend low disease activity (LDA) as initial treatment goal with remission as a subsequent target whenever possible. This study aimed to evaluate the incremental benefit of achieving remission in patients who were at LDA initially.
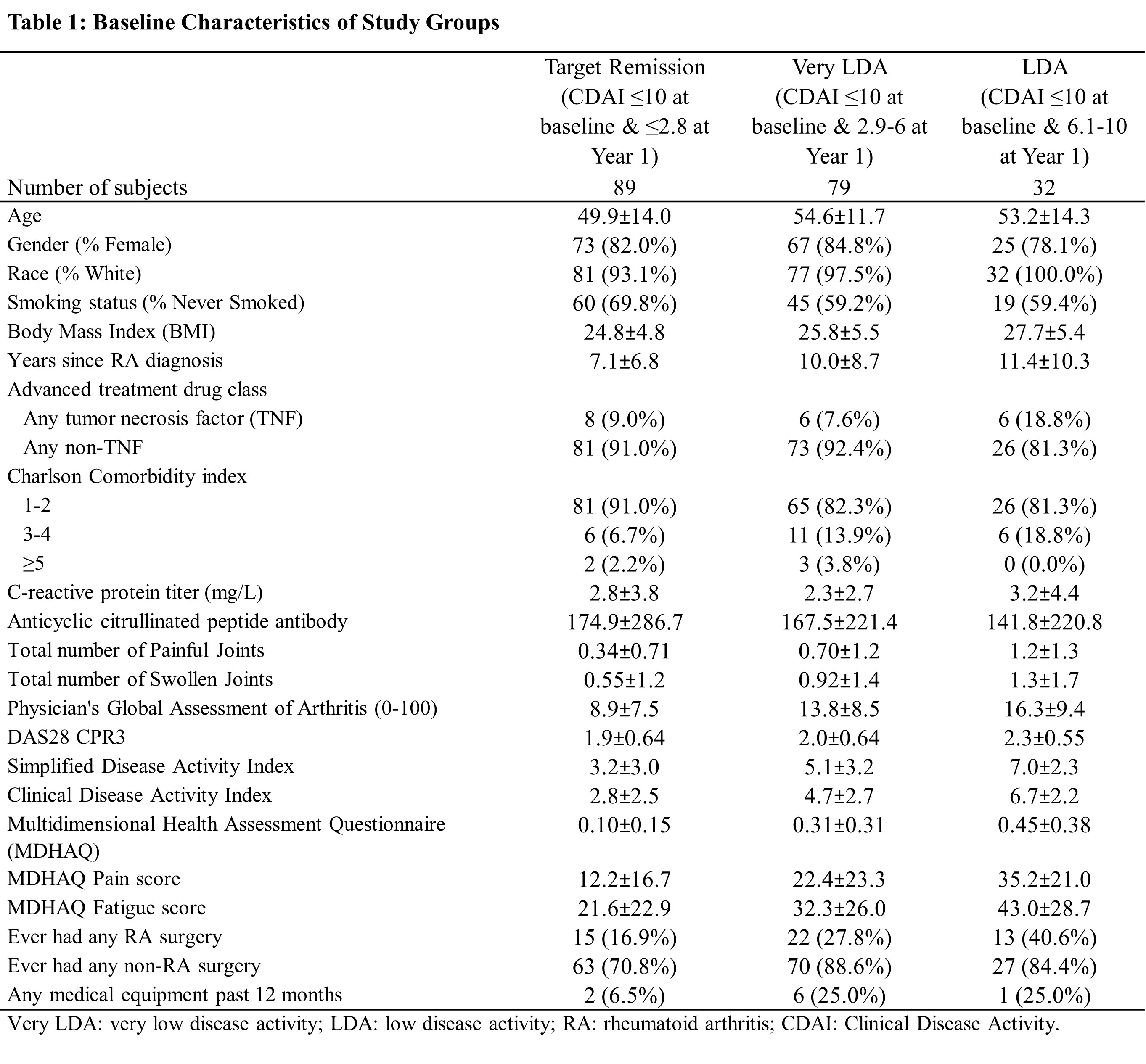
Methods: Data from the Brigham and Women’s Hospital Rheumatoid Arthritis Sequential Study (BRASS), a prospective registry of 1,581 adult RA patients with established or recent-onset RA, were analyzed. Out of 1,581 patients, 1,478 (93%) satisfied ACR 1987 criteria at baseline. 437 enrollees have been assessed using the ACR 2010 criteria and 345 (79%) patients were classified as definite RA. RA patients exposed to advanced treatment and consistently at target of LDA (Clinical Disease Activity Index [CDAI] ≤10) at baseline and after one-year follow-up were classified into 3 groups based on their CDAI at one-year follow-up: 1) Remission: CDAI ≤2.8, 2) Very LDA: CDAI >2.8 & ≤6, and 3) LDA: CDAI >6 & ≤10. Baseline was defined as the first record with evidence of exposure to biologic disease-modifying antirheumatic drugs (bDMARDs) in BRASS. PROs including Multidimensional Health Assessment Questionnaire (MDHAQ) overall, pain, fatigue scores and proportions of surgeries and durable medical equipment (DME) use in year 1 and year 2 were assessed. Multivariable regression analyses adjusted for baseline age, gender, RA duration, Charlson comorbidity index and disease activity were performed.
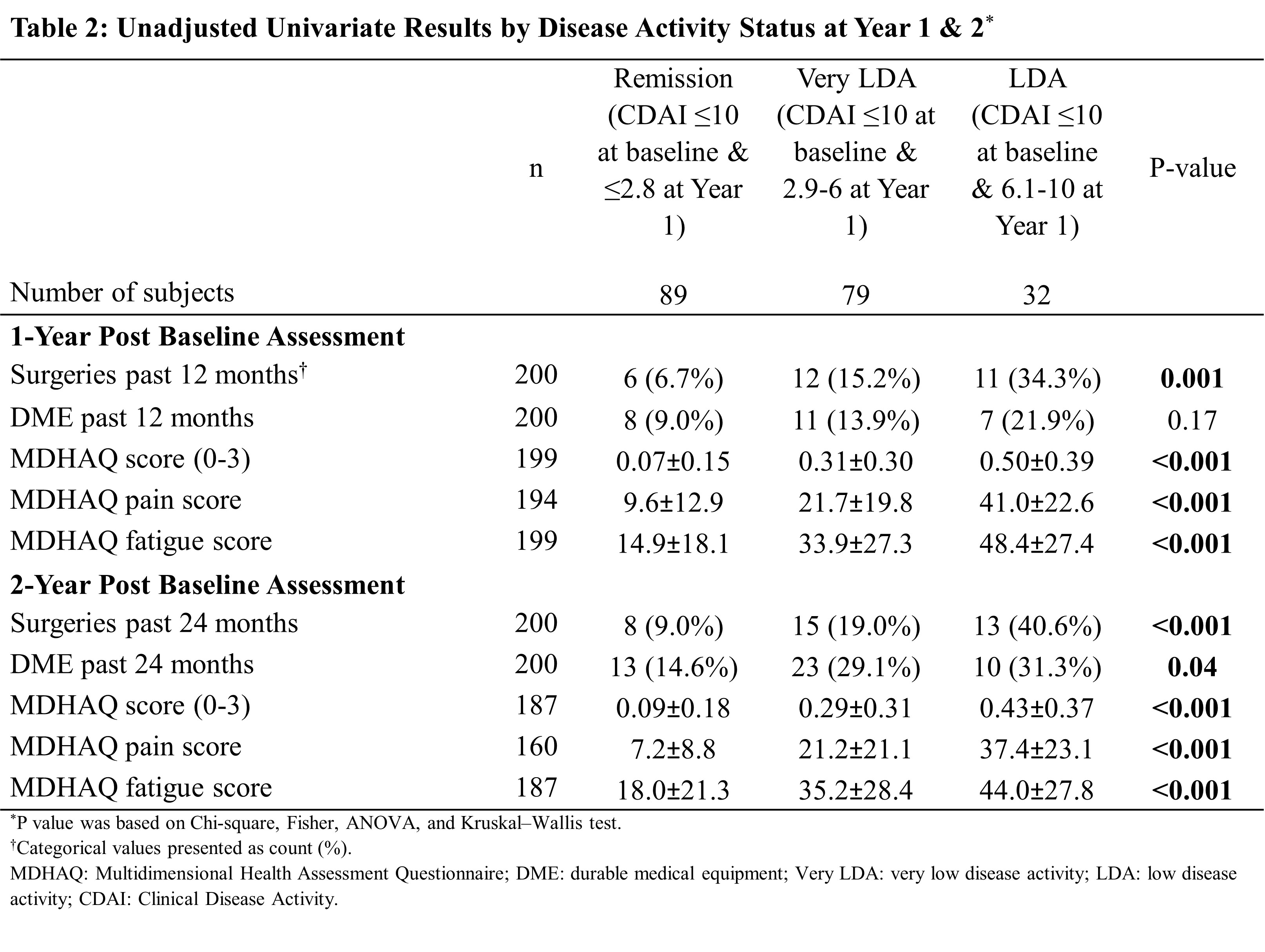
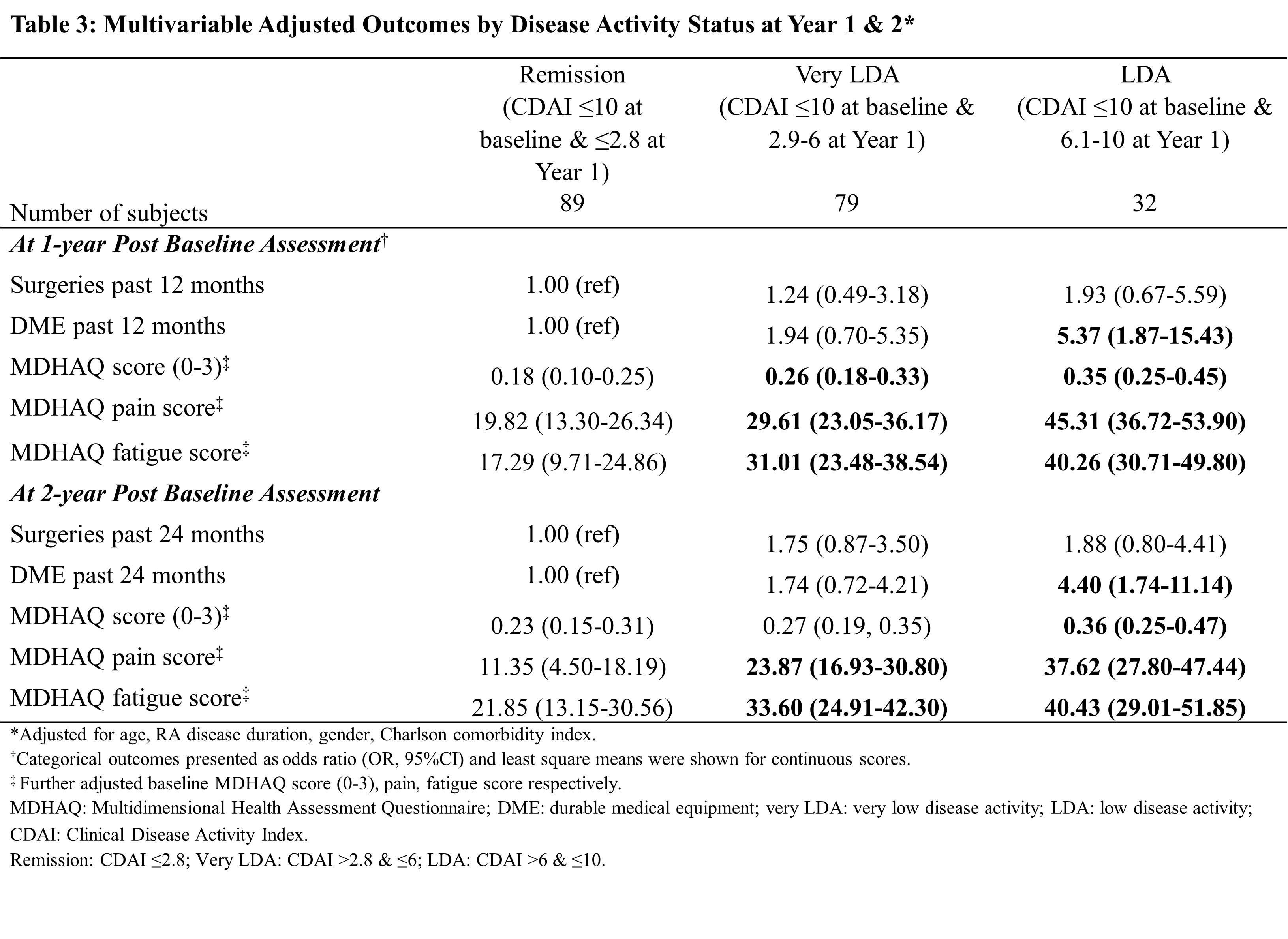
Results: A total of 637 patients received advanced therapy and had CDAI data in BRASS, of which 200 (31%) who achieved a treatment target of LDA at baseline and remained at LDA after one-year of follow-up were included in this analysis. At the end of one year, 89 (45%) patients achieved remission, 79 (40%) attained a very LDA while 32 (16%) had LDA. Patients in the remission group appeared to have lower body mass index, shorter disease duration and lower disease activity at baseline than patients who did not achieve remission. Achieving remission (CDAI ≤2.8) was associated with further reduction of DME use and better MDHAQ function scores than patients with CDAI between 6 and 10 (P≤0.001). After adjusting for potential confounders, patients with CDAI >6 & ≤10 had higher risk of DME use (OR=5.4 at year 1, OR=4.4 at year 2), higher MDHAQ overall (Δ=+0.17 at year 1, +0.13 at year 2), pain (Δ =+25.49 at year 1, +26.27 at year 2) and fatigue (Δ = +22.97 at year 1, +18.57 at year 2) scores than patients who achieved remission CDAI ≤2.8, despite no observed difference in surgery. There was no statistical difference in surgery and DME utilization among patients with very LDA compared to those achieving remission.
Conclusion: Among patients who were at initial treatment target of low disease activity (CDAI ≤10), achieving remission status was associated with better functional outcomes and lower DME use. Patients who achieved very LDA had similar surgery and DME outcomes as those in remission. New effective drugs, treatment strategies and policies that can bring more RA patients into remission offer the potential to reduce functional disability and associated economic burden to the health care system.
To cite this abstract in AMA style:
Zhao J, Sbarigia U, Kwong J, Naik C, Zazzetti F, Shadick N, Weinblatt M. What Are the Benefits of Treating Rheumatoid Arthritis Patients to Remission After Achieving Low Disease Activity in Clinical Practice? [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/what-are-the-benefits-of-treating-rheumatoid-arthritis-patients-to-remission-after-achieving-low-disease-activity-in-clinical-practice/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/what-are-the-benefits-of-treating-rheumatoid-arthritis-patients-to-remission-after-achieving-low-disease-activity-in-clinical-practice/