Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
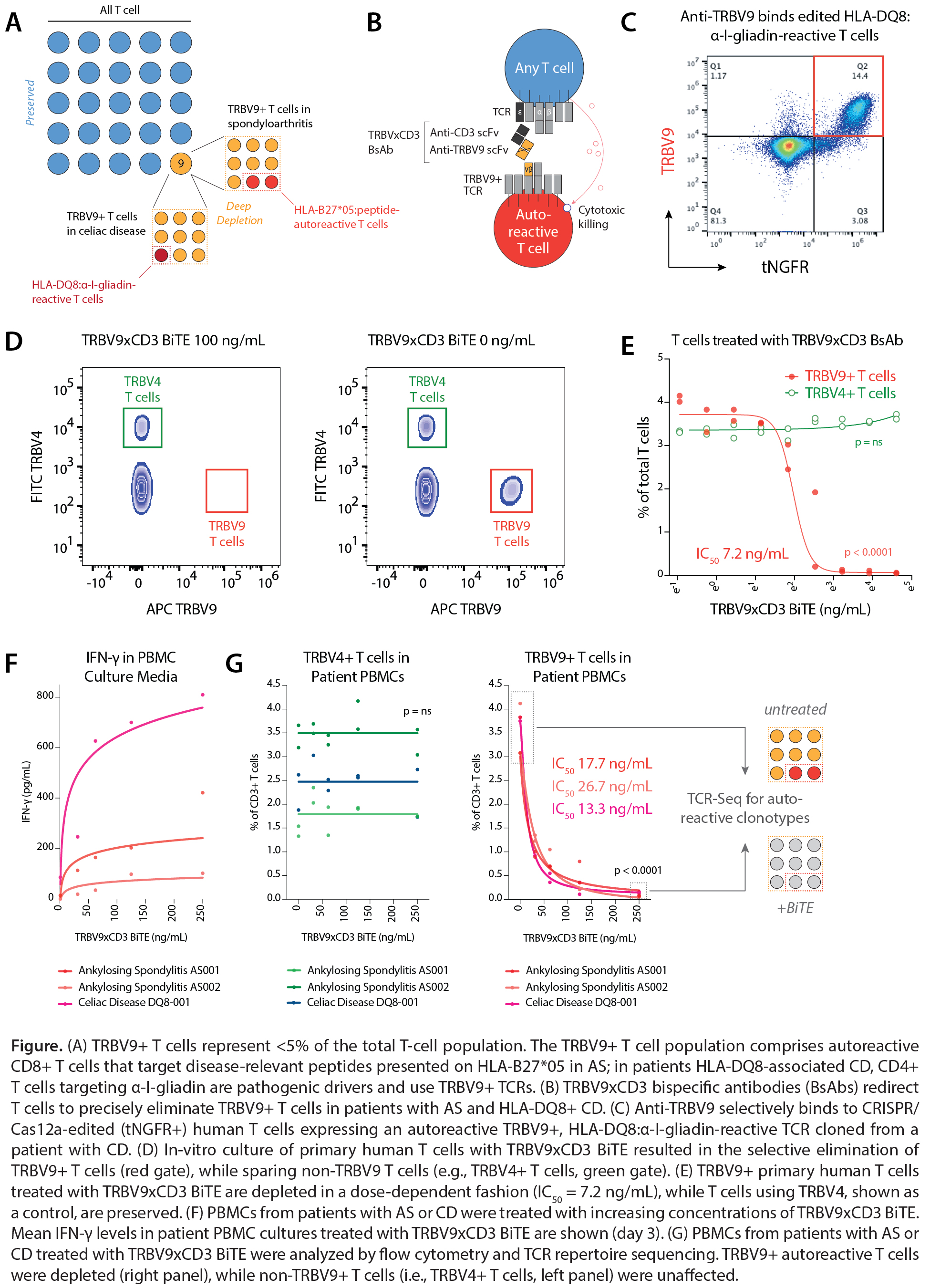
Background/Purpose: Therapies that indiscriminately deplete all T cells carry a high risk of opportunistic infection, precluding their widespread use in T cell-mediated autoimmune diseases. Targeting autoreactive T cells with selectivity, conversely, is difficult given an incomplete understanding of their antigen specificities. Germline-encoded T cell receptor (TCR) regions provide an opportunity to target autoreactive T cells, even if the cognate autoantigen is unknown. TCR β-chain variable (TRBV) gene usage by autoreactive T cells can be highly skewed by HLA alleles. In ankylosing spondylitis (AS), CD8+ T cells against disease-relevant peptides presented on HLA-B27*05 are contained in the TRBV9+ T cell fraction. Analogously, HLA-DQ8:α-I-gliadin-reactive CD4+ T cells that drive celiac disease (CD) use TRBV9 (A). Deep depletion of the TRBV9+ T cell compartment has the potential to achieve long-standing disease remission in patients with AS and CD without increasing the risk of infection. We therefore developed a precision T cell-engaging BsAb therapy that selectively eliminates TRBV9+ T cells while sparing >95% of the T cell repertoire.
Methods: Anti-TRBV9 and anti-CD3 single-chain variable fragment (scFv) sequences were synthesized, cloned, and TRBV9xCD3 BsAbs expressed as single-chain diabodies (scDbs) and bispecific T-cell engagers (BiTEs). Human T cells were CRISPR-Cas12a-edited to replace their endogenous TCRs with autoreactive, TRBV9+ TCRs cloned from patients with AS or CD. Binding of anti-TRBV9 to edited T cells was determined by flow cytometry. The potency and specificity of TRBV9xCD3 BsAbs against primary human T cells was interrogated in culture, and cytotoxicity was quantified by flow cytometry and interferon (IFN)-γ ELISA. PBMCs from patients with HLA-B27+ AS and HLA-DQ8+ CD were treated with BsAbs, and selective depletion of TRBV9+ T cells quantified by flow cytometry and TCR repertoire sequencing.
Results: We developed BsAbs to redirect T cells to selectively eliminate TRBV9+ T cells that harbor the autoreactive T cell compartment in patients with AS and HLA-DQ8+ CD (B). Anti-TRBV9 bound engineered human T cells expressing autoreactive TRBV9+ TCRs cloned from patients with AS or CD, but not T cells expressing other TRBV alleles (C). In culture of primary human T cells, TRBV9xCD3 BiTEs resulted in the selective elimination of TRBV9+ T cells in a dose-dependent manner (IC50: 7.2 ng/mL) (p< 0.0001), sparing T cells with other TRBV gene usage (e.g., TRBV4+ T cells) (p=ns) (D-E). Treatment of PBMCs from patients with AS and HLA-DQ8+ CD with TRBV9xCD3 BiTEs induced limited IFN-γ release (F) and depleted of TRBV9+ T cells (IC50: 13-27 ng/mL) and disease-relevant autoreactive T cell clonotypes (p< 0.0001), but not non-TRBV9 T cells (p=ns) (G).
Conclusion: We describe an off-the-shelf, precision immunotherapy approach for the deep depletion of autoreactive TRBV9+ T cells in HLA-B27+ AS and HLA-DQ8+ CD. TRBV9xCD3 BsAbs are highly potent and specific at eliminating TRBV9+ T cells while sparing other T cell populations. Different to monoclonal antibodies targeting TRBV9, BsAbs may offer an opportunity to achieve sustained depletion and reset of the autoreactive T cell compartment in AS and CD.
To cite this abstract in AMA style:
Glavaris S, Pearlman A, Liu J, Ge J, Xia Y, Kaeo K, Awosika T, Gliech C, Nichakawade T, Marcou N, Bettegowda C, Pardoll D, Kinzler K, Zhou S, Vogelstein B, Paul S, Konig M. TRBV9-Targeted Bispecific T Cell-Engaging Antibodies to Reset the Autoreactive T Cell Compartment in Spondyloarthritis and HLA-DQ8 Celiac Disease [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/trbv9-targeted-bispecific-t-cell-engaging-antibodies-to-reset-the-autoreactive-t-cell-compartment-in-spondyloarthritis-and-hla-dq8-celiac-disease/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/trbv9-targeted-bispecific-t-cell-engaging-antibodies-to-reset-the-autoreactive-t-cell-compartment-in-spondyloarthritis-and-hla-dq8-celiac-disease/