Session Information
Date: Sunday, November 17, 2024
Title: Abstracts: Innate Immunity: Molecular Insights Into Immune Dysregulation
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Antiphospholipid antibodies (aPL) confer a high risk for adverse pregnancy outcomes, especially in women with SLE. aPLs can induce pro-inflammatory signaling via TLR8 receptors, but mouse models to study the role of TLR8 are limited due to the attenuated ssRNA-binding capacity of mouse TLR8. We generated a spontaneous model of aPL-induced pregnancy loss by crossing Sle1 mice with mice expressing a human TLR8 transgene (Sle1.huTLR8tg). Sle1.huTLR8tg mice but not Sle1 or C57BL6.huTLR8tg mice have a high frequency of pregnancy loss, demonstrating the requirement for both autoantibodies and TLR8 in fetal-placental injury.
Methods: Bulk RNA-sequencing was conducted on feto-placental units from Sle1 and Sle1.huTLR8tg mice at Day E8.5 (day of peak decidualization and spiral artery remodeling) and Day E13.5 (at which time the placenta has been fully formed). qPCR was performed to validate RNAseq findings. Uterine natural killer (uNK) cells were identified at Day E9.5 on flow cytometry as Lin- NK1.1+ cells, and further classified into tissue-resident (CD49a+) or circulating (CD49b+) subsets. DBA lectin was used to identify uNK cells on IHC. Placental myeloid cell subsets were analyzed in the same tissues by flow cytometry. Sle1.huTLR8tg to Sle1 bone marrow chimeras were generated and placentas from pregnant chimeras were analyzed for myeloid subsets by flow cytometry.
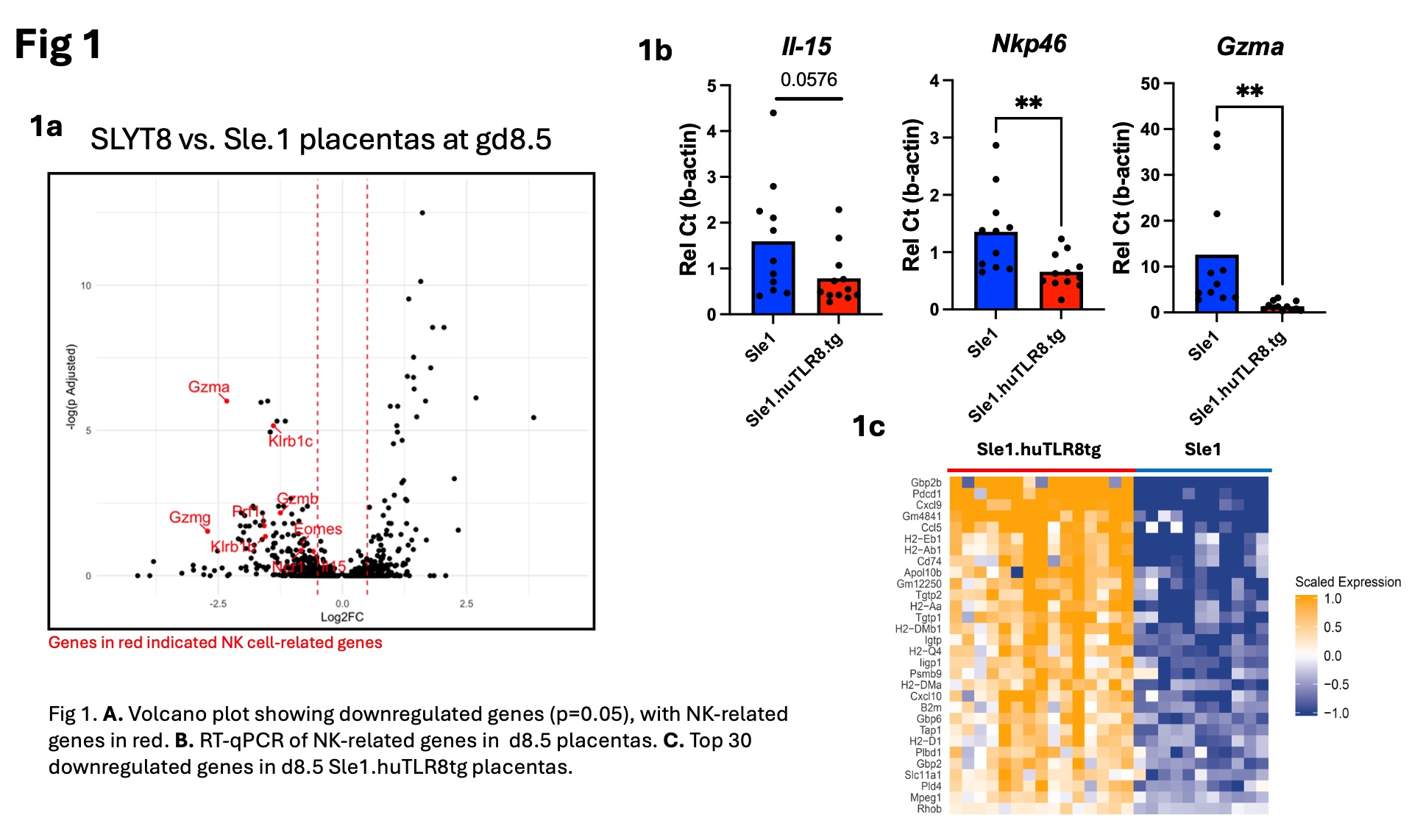
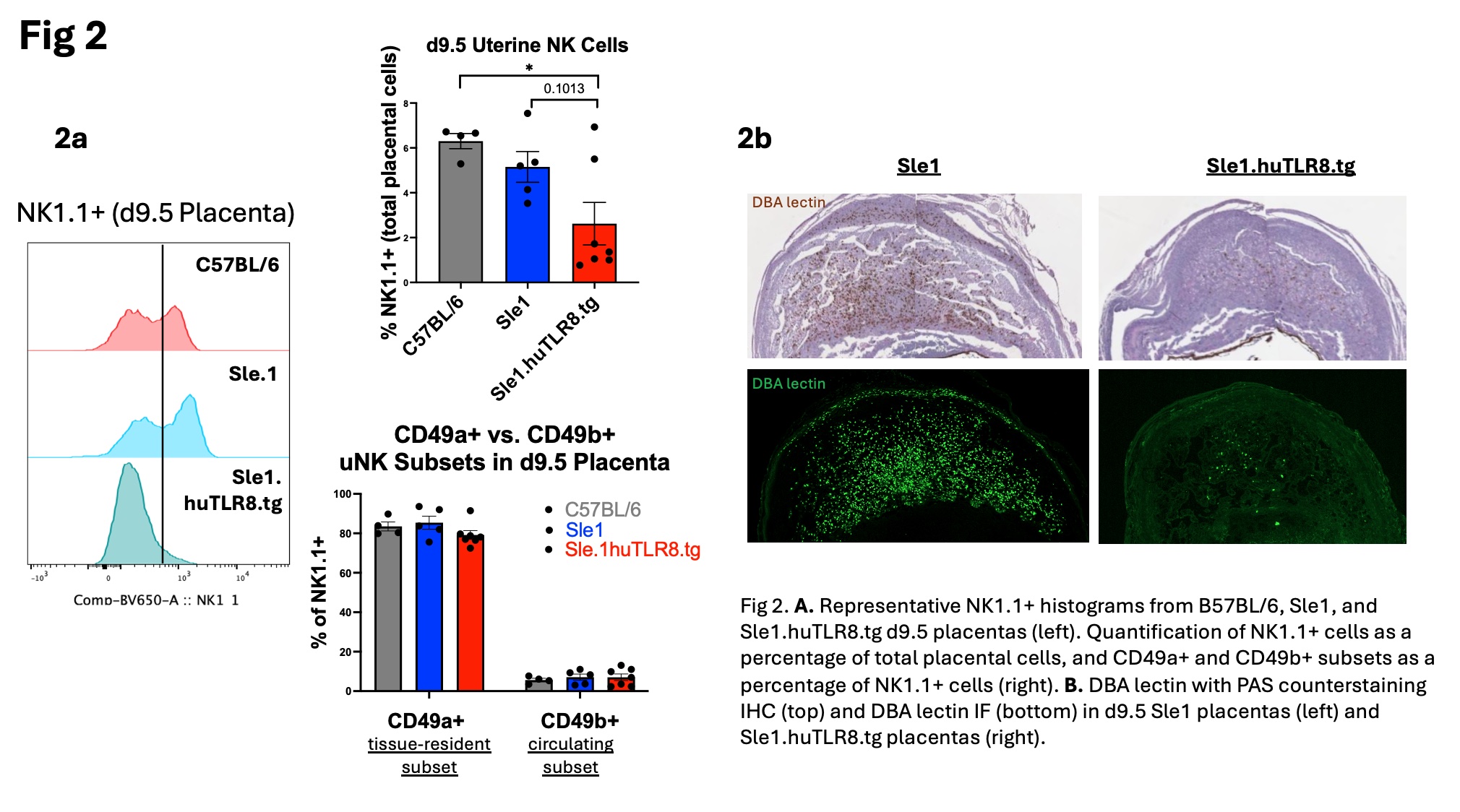
Results: Placental abnormalities in Sle1.huTLR8tg mice include attenuation of vessels in the labyrinth, thinning of the junctional zone, thickened arterial walls, placental infarcts and inflammation. Bulk RNA sequencing of Sle1.huTLR8tg placentas at Days E8.5 and E13.5 showed a suppressed uNK cell gene signature, confirmed by qPCR (Fig. 1b). Flow cytometry (Fig. 2a) and IHC (Fig. 2b) demonstrated a decrease in uNK cell number in Day E9.5 Sle1.huTLR8tg feto-placental units with no difference in the frequency of tissue-resident vs. circulating uNK subsets. Concurrently, an upregulation of myeloid-related genes was observed at Day E8.5 (Fig 1c). A continued decrease in placental NK cells and thinning of the junctional zone were evident at Day E13.5. Analysis of placentas from bone marrow chimeras indicated preferential recruitment of huTLR8tg myeloid cells to the placentas.
Conclusion: Sle1.huTLR8tg mice are the first spontaneous model for aPL-induced pregnancy loss and demonstrate early placental developmental defects including a loss of uNK cells and junctional zone trophoblasts, the major cell types involved in placental spiral artery remodeling. An increase in placental myeloid cells in Sle1.huTLR8tg placentas may indicate an upstream regulatory defect affecting uNK cell proliferation and/or recruitment. Similar immune defects have recently been described in 1st trimester human APS placentas. Subsequent compromise of the placental vasculature leads to the classical features of placental infarction and inflammation. Our findings suggest a possible role for human TLR8 in aPL-induced pregnancy loss with potential therapeutic implications. Further studies are needed to understand the factors regulating uNK cell function and recruitment to the placenta, including a potential role for myeloid cells.
To cite this abstract in AMA style:
Xia Y, Maria N, Yi Z, Raparia C, Konanur Gopikrishna G, Zhang W, Davidson A. Placental Developmental Defects in a Humanized-TLR8 Mouse Model of Spontaneous Anti-Phospholipid Antibody Induced Pregnancy Loss [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/placental-developmental-defects-in-a-humanized-tlr8-mouse-model-of-spontaneous-anti-phospholipid-antibody-induced-pregnancy-loss/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/placental-developmental-defects-in-a-humanized-tlr8-mouse-model-of-spontaneous-anti-phospholipid-antibody-induced-pregnancy-loss/