Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Early diagnosis and treatment of SLE improves prognosis and quality of life.1 Belimumab (BEL), a human immunoglobulin G1λ (IgG1λ) mAb that selectively binds to and inhibits soluble B-lymphocyte stimulator, has real-world effectiveness for SLE.2 Patients (pts) with shorter disease duration and lower organ damage have a greater probability of favorable outcomes with BEL treatment; earlier treatment may be beneficial.3 European Alliance of Associations for Rheumatology (EULAR) 2023 recommend immunosuppressant (IS) use is not necessary before biologic initiation.1 This study aims to compare clinical outcomes in BEL-treated pts with and without prior IS use.
Methods: This retrospective, longitudinal study (Study 217537) used de-identified claims data from the Komodo Health Database from January 2015–October 2023. Eligible pts had ≥1 claim for BEL January 2017–October 2023 (index date) and ≥24 months of continuous eligibility pre-index (12-month IS washout period followed by 12-month IS identification period). In the absence of clinical and laboratory data to apply ACR classification criteria for disease, claims-based ICD-9/10 diagnoses prior to BEL initiation determined eligibility. Pts were stratified by IS use: no IS use (0IS) vs one IS (1IS) use in the 12 months pre-index. Pts with IS use during the 12-month IS washout period or use of ≥2 IS during the 12 months pre-index were excluded. Baseline period was 12 months prior to first IS treatment date (1IS) or first new non-IS SLE treatment or oral corticosteroid (OCS) escalation date (0IS). Follow-up period ceased with the end of continuous eligibility or data availability. OCS discontinuation/reduction and rate of SLE flares were compared using Cox proportional hazards and Poisson regression models, respectively. Inverse probability of treatment weighting (IPTW) was used to adjust for confounding. Variables with a standardized difference >10% were included in regression models for a robust approach.
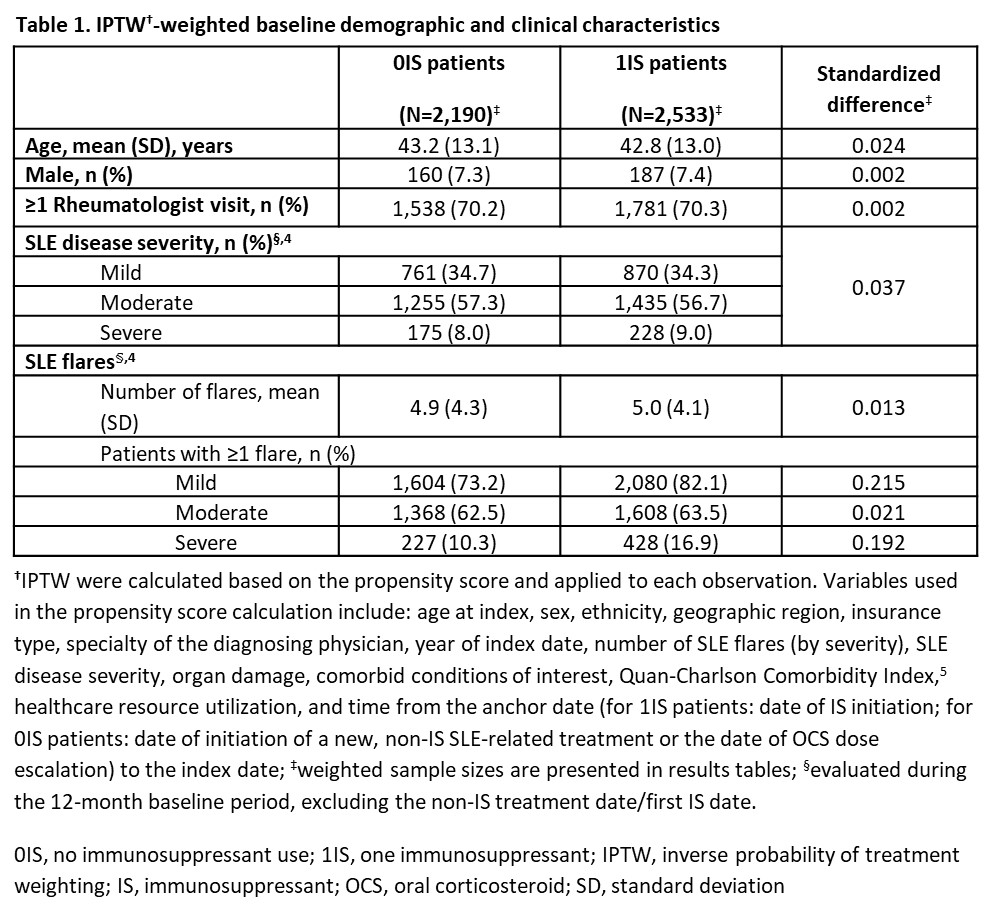
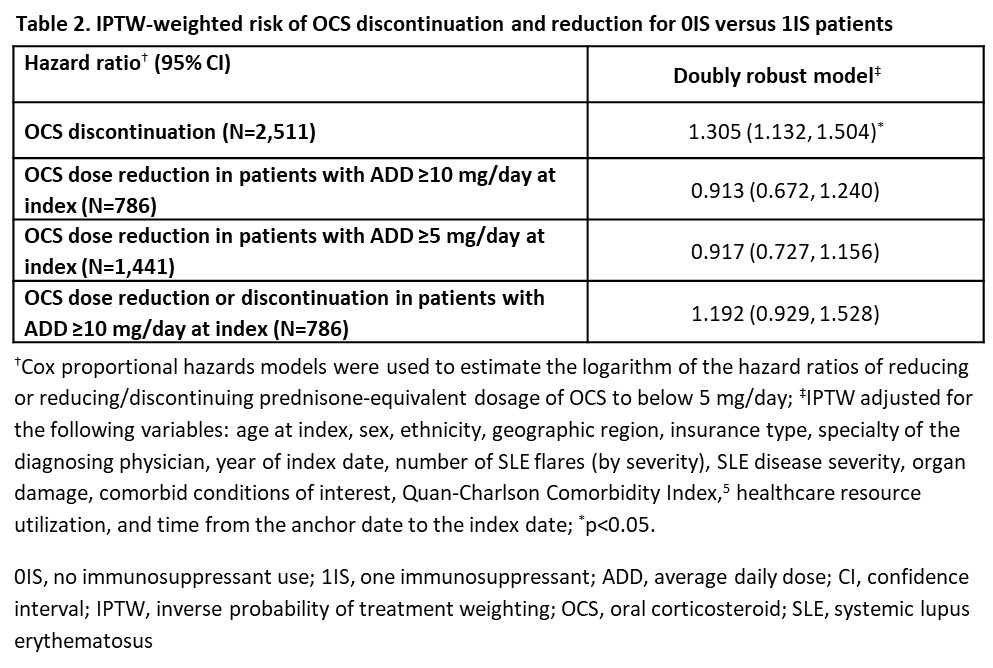
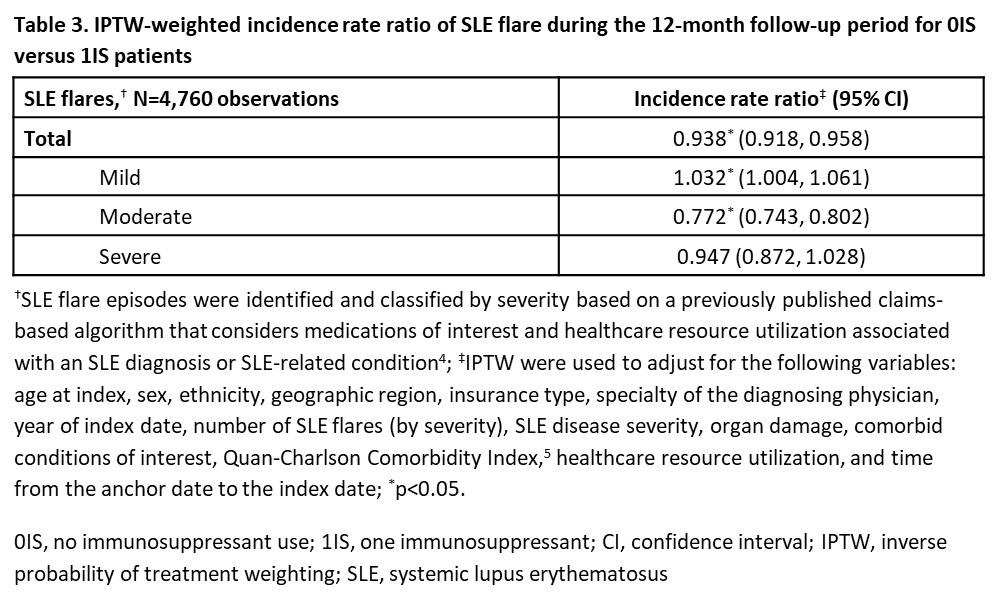
Results: There were 2,221 0IS pts and 2,539 1IS pts. Few baseline characteristics had a standardized difference >10% after applying IPTW (Table 1). 0IS and 1IS pts with index OCS use had median (95% confidence interval, CI) time to OCS discontinuation of 9.8 (8.2, 12.2) and 11.7 (10.5, 13.4) months, respectively. 0IS pts were 31% more likely to discontinue OCS (hazard ratio: 1.31; 95% CI: 1.13, 1.50; p< 0.05; Table 2). Time to OCS dose reduction and dose reduction/discontinuation were not significantly different between 0IS and 1IS pts (Table 2). 0IS pts had 6% lower rate of total SLE flares vs 1IS pts (incidence rate ratio [IRR]: 0.94; 95% CI: 0.92, 0.96; p< 0.05; Table 3). This was driven by a 23% reduction in the rate of moderate flares (IRR: 0.77; 95% CI: 0.74, 0.80; p< 0.05; Table 3).
Conclusion: Pts initiating BEL before vs after IS use were more likely to discontinue OCS and have lower rates of SLE flares, showing benefits of earlier BEL use in line with EULAR recommendations.1 This study supports clinician decision-making regarding earlier positioning of BEL within the SLE treatment pathway; pts treated with BEL before IS use had more favorable clinical outcomes.
Disclosures: M. DerSarkissian: Analysis Group, 3, GSK, 5; Y. Chen: Analysis Group, 3, GSK, 5; B. Rabideau: Analysis Group, 3, GSK, 5; T. Man: Analysis Group, 3, GSK, 5; K. Worley: GSK, 3, 11; B. Rubin: GSK, 3, 11; K. Costenbader: Amgen, 2, 5, AstraZeneca, 5, Bain HealthSciences, 2, Bristol-Myers Squibb(BMS), 2, Cabaletta Bio, 2, 5, Exagen, 5, Gilead, 5, Glaxo Smith Kline, 2, 5, Merck, 5; S. Lim: Accordant, 2, AstraZeneca, 2, Biogen, 5, BMS, 5, Gilead, 5, GSK, 2, Novartis, 5, UCB, 5.
To cite this abstract in AMA style:
DerSarkissian M, Chen Y, Rabideau B, Man T, Worley K, Rubin B, Costenbader K, Lim S. Belimumab-Treated Patients with Systemic Lupus Erythematosus Without Prior Immunosuppressant Use Have More Favorable Clinical Outcomes Than Those with Prior Use of an Immunosuppressant [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/belimumab-treated-patients-with-systemic-lupus-erythematosus-without-prior-immunosuppressant-use-have-more-favorable-clinical-outcomes-than-those-with-prior-use-of-an-immunosuppressant/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/belimumab-treated-patients-with-systemic-lupus-erythematosus-without-prior-immunosuppressant-use-have-more-favorable-clinical-outcomes-than-those-with-prior-use-of-an-immunosuppressant/