Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Sjögren’s disease (SjD) is a systemic autoimmune disease that manifests as glandular dryness and systemic symptoms. Salivary gland epithelial cells (SGEC) are pivotal in SjD pathogenesis, being both targets and active actors via interactions with immune cells. Current in vitro models fail to capture salivary glands complexity, underlining the need for innovative models that replicate epithelium/immune cell interactions.
Our objectives consisted of three axes: first, to develop and characterize differentiated salivary gland organoids (DSGO) from minor salivary gland biopsies (MSGB) of SjD and controls; second, to validate the DSGO functionality and establish analytical readouts to measure it; and third, to assess drug efficacy on DSGO.
Methods: We included SjD patients meeting the ACR/EULAR 2016 criteria, along with controls exhibiting sicca syndrome without autoimmune conditions. MSGBs were dissociated, then enclosed in an extracellular matrix (ECM), and cultured in expansion phase for organoid formation. Organoids were regularly passaged before transitioning to differentiation phase in ECM. Eventually, using ultra-low attachment 96 well plates yielded distinct differentiated organoids.
SGEC markers were examined by immunohistochemistry and RT-qPCR post-differentiation, and RNAseq analysis was performed. Further, swelling assay was conducted to analyze organoid functionality, enabling response measurements via live-cell imaging.
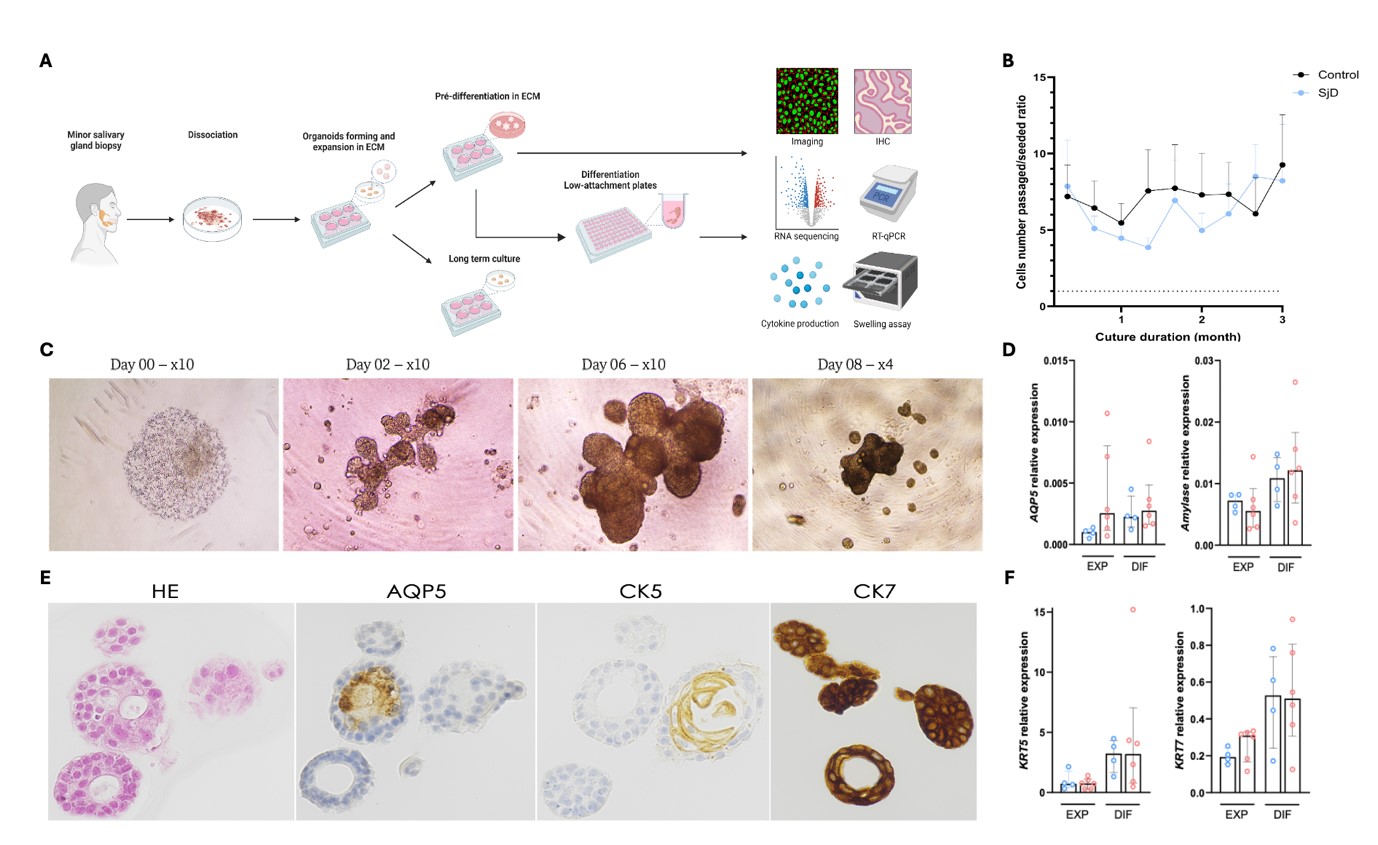
Results: We included 11 SjD patients and 17 controls. In both groups, organoid formation and differentiation were observed, with an average culture period of 3.5 ± 1.7 months (Figure 1A-B). DSGOs showed highly differentiated aspects, featuring acinar-like structures, and branching (Figure 1C).
We then characterized the DSGOs, they exhibited ductal markers (CK5/CK7) and acinar markers (AQP5, α-amylase) (Figure 1D-F).
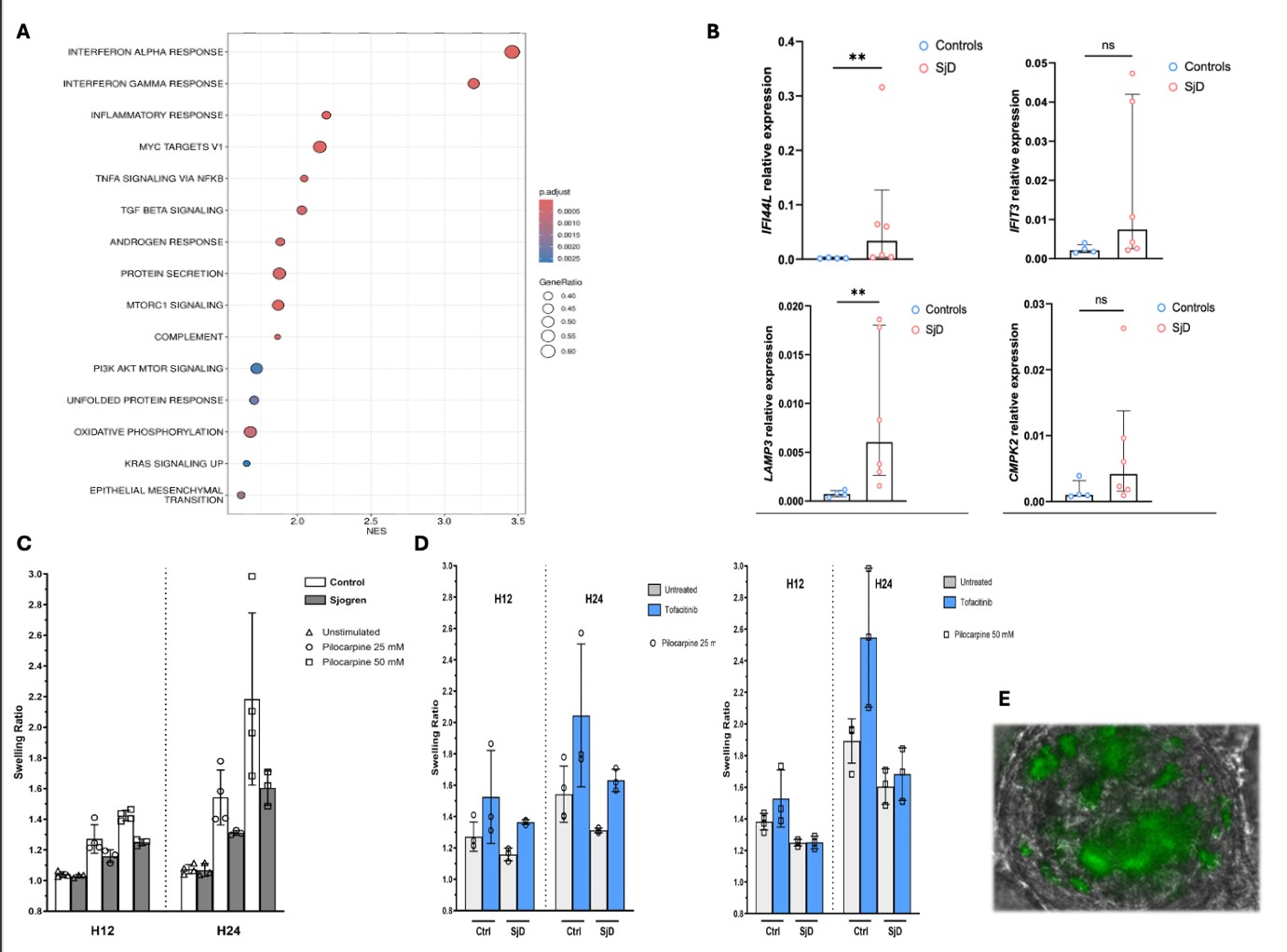
We evaluated the swelling capacity by exposing DSGOs to pilocarpine, a cholinergic agonist. Induced swelling ability notably increased in DSGOs indicating their capacity for secretory activities. Swelling capacity was reduced in SjD organoids, mirroring the disease phenotype (Figure 2C).
Through RNAseq, we noted that DGSOs from SjD display a distinct transcriptomic profile, including an enduring IFN signature compared to controls (Figure 2A-B), in the absence of any external stimulation. Thus, we opted to block this pathway by JAK inhibitor.
Exposure to tofacitinib (anti JAK1/3) enhanced the swelling ability, suggesting the role of JAK/STAT pathway in inducing secretion and therapeutic efficacy (Figure 2D).
Besides, early trials aimed at developing immune organoids by introducing B cells to DSGO were conducted. (Figure 1E).
Conclusion: We established a culture protocol to generate DSGOs from MSGB of both SjD and controls which allows long-term expansion and differentiation. Also, a multi-channel replicable 3D culture system was created for high-throughput multi-drug tests and analysis on DSGOs.
Experiments are in progress for adding immune cells in organoids to assess the impact of immune infiltration on epithelial functions and to test the effect of drugs on the system.
To cite this abstract in AMA style:
Goudarzi N, Meudec L, Pascaud J, Jaulin F, Pascal Q, Lazure T, E Silva Saffar S, Mariette X, Nocturne G. Development and Functional Characterization of Salivary Gland Organoids to Investigate Sjögren’s Disease [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/development-and-functional-characterization-of-salivary-gland-organoids-to-investigate-sjogrens-disease/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-and-functional-characterization-of-salivary-gland-organoids-to-investigate-sjogrens-disease/