Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Cannabis and its derivatives are increasingly integrated into treatments for rheumatic diseases, yet there is a significant lack of evidence and systematic data collection regarding their use and impact. As the prevalence and diversity of cannabinoid-containing products rise globally, gathering useful Real-World Data (RWD) becomes increasingly complex. The National Institute on Drug Abuse (NIDA) has emphasized the urgent need for harmonizing data collection. Additionally, Health Care Professionals (HCPs) often hesitate to record cannabis use data due to perceived legal challenges. This lack of systematic learning and documentation hinders understanding of the benefits, risks, and potential Drug-Drug Interactions (DDIs) associated with these products, which is crucial for informed healthcare decisions in rheumatic disease management. This abstract addresses the unmet educational needs and improve HCPs’ knowledge, attitudes (K/A), and data collection practices related to Cannabis and Cannabis-Derived Products (CCDP), including hemp and hemp-derived products.
Methods: We conducted a preliminary survey with HCPs, evaluating their K/A of CCDPs through a 40-question survey. Building on these insights, we developed a pilot study that included an updated K/A survey integrated with the Cerium educational platform. The Cerium platform features four educational modules, blogs, a virtual journal club, and discussions on patient-reported outcomes (PROs). Budsinfo, a systematic data collection tool, was integrated to report and analyze real-time outcomes related to CCDP use from participating HCPs. Follow-up surveys and discussions are conducted at virtual monthly meetings to ensure continuous learning and adaptation.
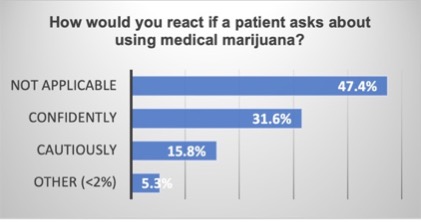
Results: Preliminary findings show that 90.9% of HCPs are open to using tools to assess patients’ cannabis experiences. However, 57.89% found the use of a self-reported Substances Use Scale App “Not applicable,” indicating barriers such as lack of access to technology and training. Additionally, 63.16% found encouraging patients to use an adverse effect reporting tool “Not applicable,” highlighting systemic barriers in patient engagement. Most HCPs rely on news and social media for cannabis information, while preferring lectures and medical journals for other new medications. Notably, over 57% of respondents reported a lack of confidence in their out current knowledge of cannabinoid molecules, underscoring the need for enhanced education and systematic data collection. And over 47% responded “not applicable” to the question, “How would you react if a patient asks you about using medical marijuana?”
Conclusion: Budsinfo and Cerium constitute a potentially comprehensive solution to bridge critical gaps in adverse event reporting and promote data-driven decision-making for regulatory agencies, HCPs, and the public. This holistic approach, combining real-time data collection with dedicated educational resources, contributes to enhanced product safety understanding and consumer health in rapidly evolving markets. By implementing these tools, we can better support healthcare providers, ultimately improving patient care and outcomes.
To cite this abstract in AMA style:
Marcu J, Molloy P, Pray K, Simon T. Addressing Unmet Needs in Cannabis and Hemp by Improving Knowledge and Data Collection [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/addressing-unmet-needs-in-cannabis-and-hemp-by-improving-knowledge-and-data-collection/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/addressing-unmet-needs-in-cannabis-and-hemp-by-improving-knowledge-and-data-collection/