Session Information
Date: Sunday, November 17, 2024
Title: RA – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Janus kinase inhibitors (JAKi) are a class of disease activity-modifying drugs (DMARDs) that have quickly established a role in managing rheumatoid arthritis. Inhibition of the JAK/STAT pathway makes them potent immunosuppressants with comparable efficacy to antibodies and other biologicals. However, this mechanism of action has been linked to several adverse effects, including infection, bowel perforation, and cardiovascular events. We investigated the association between JAKi use and major adverse events leveraging the large TriNetX database.
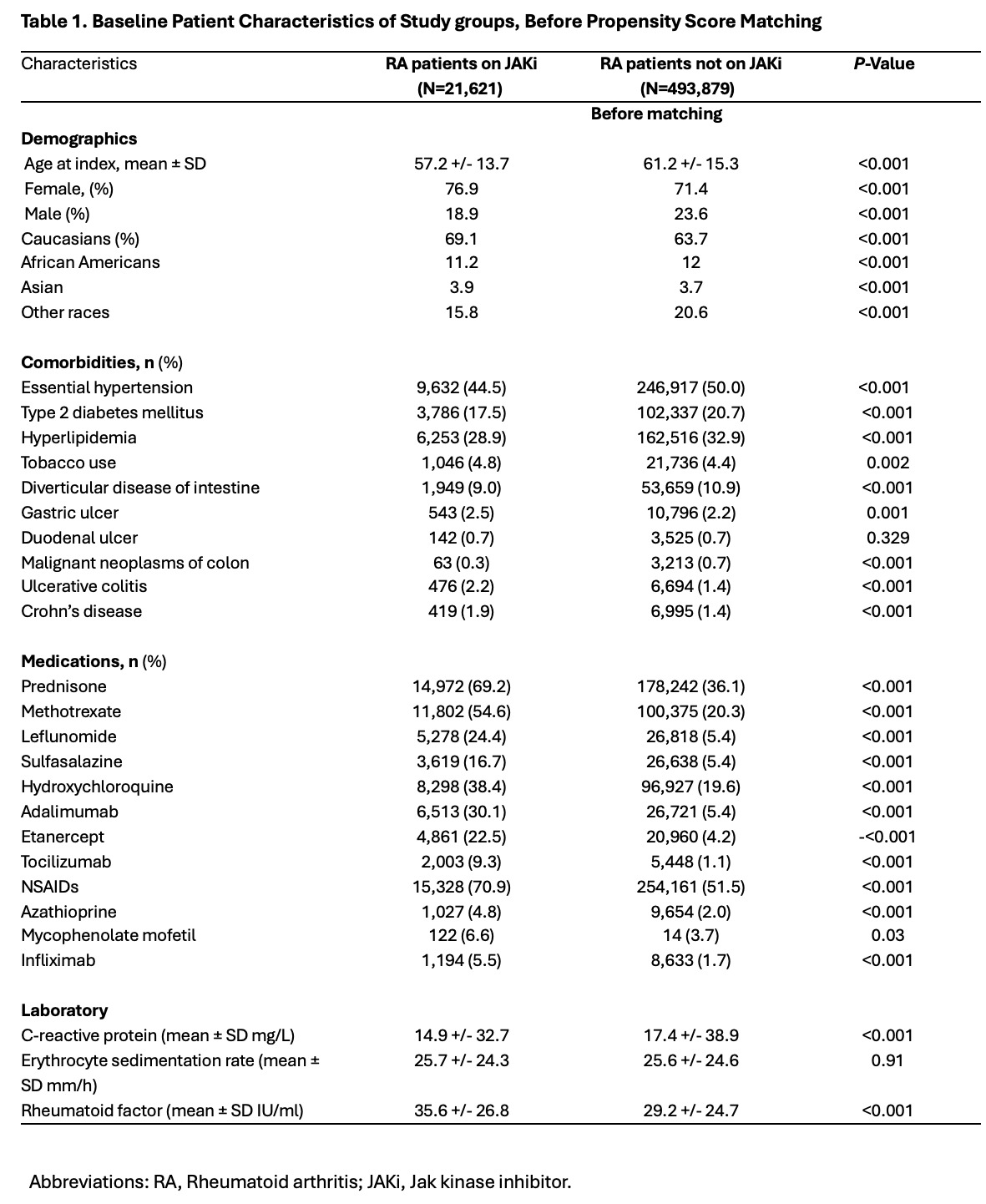
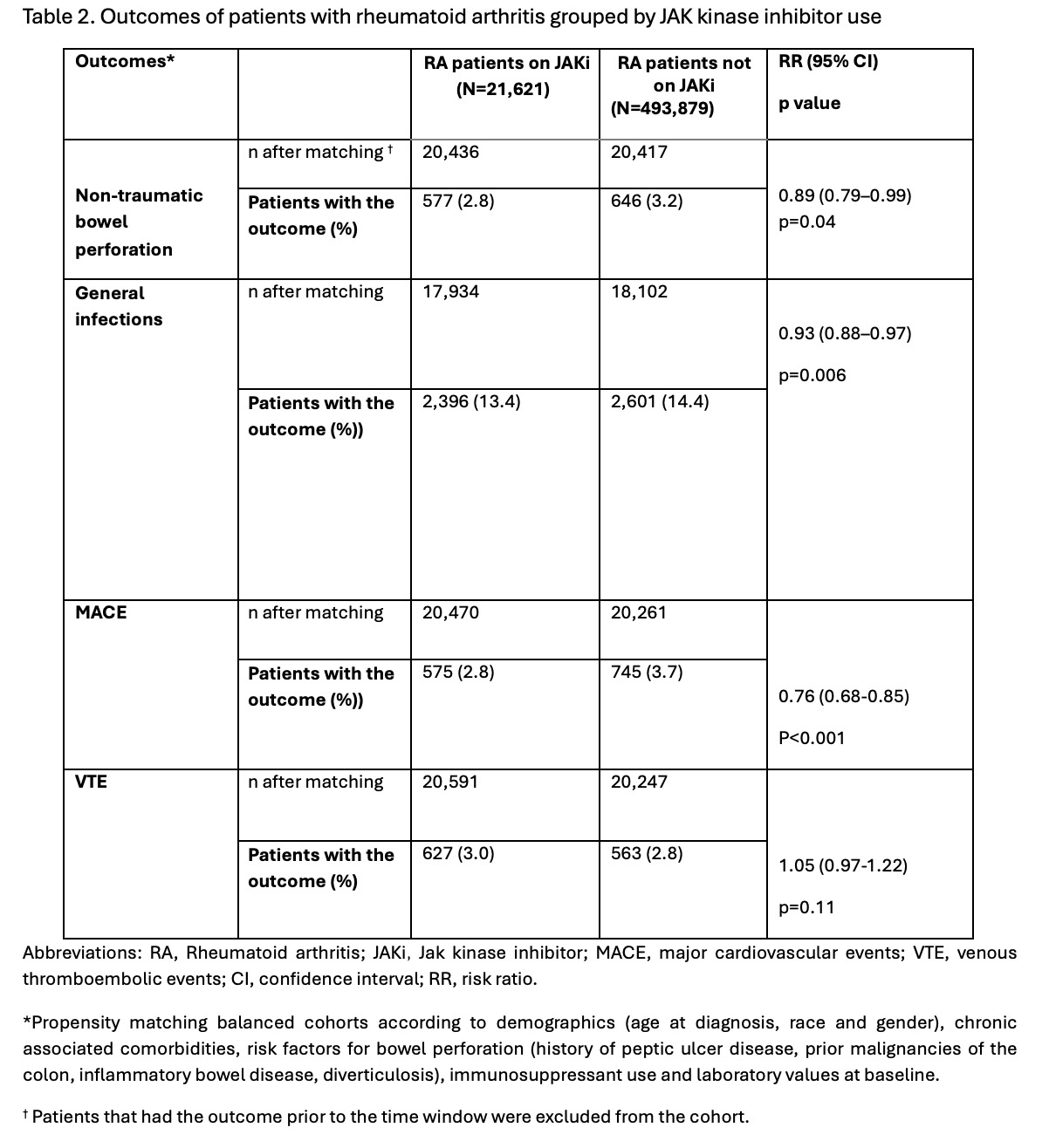
Methods: This is a nationwide, retrospective study. We used the global, multicenter research network (TriNetX) database from 89 large healthcare organizations across multiple countries. We included patients over 18 years with RA diagnosed between January 1, 2020, and June 9, 2024, using ICD-10 and ICD-9 codes. Patients were compared based on the use of JAKi (tofacitinib or baricitinib or upadacitinib) using 1:1 propensity score matching. Matching accounted for demographics (age at diagnosis, race, and gender), chronic associated comorbidities, risk factors for bowel perforation (history of peptic ulcer disease, prior malignancies of the colon, inflammatory bowel disease, diverticulosis), immunosuppressant use and laboratory values at baseline. Outcomes included non-traumatic bowel perforation, general infections, major cardiovascular events (MACE), and venous thromboembolic events (VTE). Exposure for outcomes was anytime post-index event, excluding patients with pre-index event outcomes. Risk ratios (RR) with 95% confidence intervals were calculated for each outcome, with a p-value < 0.05 considered significant.
Results: We identified 21,621 RA patients on JAKi and 493,879 RA patients not on JAKi. The mean age at diagnosis was 57.2 ± 13.7 for patients on JAKi and 61.2 ± 15.3 years for patients not on JAKi. In both groups of RA patients, females (76.9% and 71.4%) and Caucasians (69.1% and 63.7%) were the most common gender and race, respectively. Comorbidities like (hypertension, type 2 diabetes, hyperlipidemia, diverticular disease, and neoplasms of the colon) were more common in RA patients not on JAKi. Conversely, JAKi users were more frequently obese smokers, had a higher use of immunosuppressant medications, and higher levels of C-reactive protein (CRP) and rheumatoid factor (RF) at baseline. After matching, RA patients on JAKi had lower risk of bowel perforation (RR=0.89, CI95% 0.79, 0.99; p=0.04), general infections (RR= 0.93, CI95% 0.88,0.97; p=0.006), and MACE (RR= 0.76, CI95% 0.68,0.85; p< 0.001) compared to RA patients not on JAKi. No significant risk difference was found in VTE events between groups.
Conclusion: In this nationwide retrospective study, we found that RA patients on JAKi had reduced risk of bowel perforation, general infections, and MACE- a surprising finding contrary to most reported literature. While no mechanistic explanation can be determined from our study design, we hypothesize that JAK/STAT inhibition compares favorably to other immunosuppressants and treatments for patients not on JAKi, including NSAIDs.
To cite this abstract in AMA style:
Gonzalez Moret Y, Lema D, Rodriguez Quinonez F. Nationwide Retrospective Cohort Study on Clinical Outcomes in Rheumatoid Arthritis Patients Treated with JAK Inhibitors [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/nationwide-retrospective-cohort-study-on-clinical-outcomes-in-rheumatoid-arthritis-patients-treated-with-jak-inhibitors/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/nationwide-retrospective-cohort-study-on-clinical-outcomes-in-rheumatoid-arthritis-patients-treated-with-jak-inhibitors/