Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Patients with rheumatic diseases face illness-related uncertainty managing ambiguous and unpredictable symptoms. Such uncertainty is associated with decreased emotional and physical well-being (Wallace et al., 2021). Current uncertainty measurements are long, restricting routine implementation. We aimed to 1) develop and validate a brief measure of rheumatic-illness related uncertainty; and 2) explore sources of uncertainty in people with systemic vasculitis.
Methods: Patients with ANCA-associated vasculitis (AAV), IgG4-related disease (IgG4-RD), and systemic sclerosis (SSc) completed surveys assessing uncertainty (rheumatology version of the Mishel Uncertainty in Illness Scale, Survivor Version; MUIS-S), anxiety (GAD-7), depression (PHQ-8), sickness impact (SIP), and sleep duration. First, we performed an exploratory factor analysis (EFA) of the MUIS-S to identify the variance across each item following the established convention of 0.6 (stopping rule of 75% variance explained per factor) to select the final items. Second, we tested the brief uncertainty measure for internal consistency (Cronbach’s alpha) and convergent validity (Pearsons’s correlation coefficient). Third, we used hierarchal regression models to assess variance explained by uncertainty. Fourth, patients with vasculitis from the Vasculitis Patient-Powered Research Network (VPPRN) completed the brief measure and a confirmatory factor analysis (CFA) was performed. Fifth, VPPRN participant open-ended responses describing their primary sources of uncertainty were assessed in a qualitative analysis with an iterative hybrid framework using deductive and inductive reasoning.
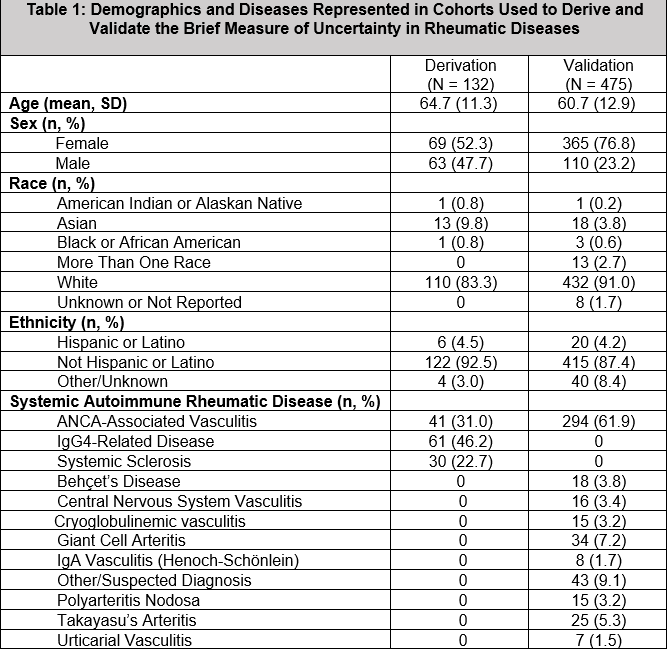
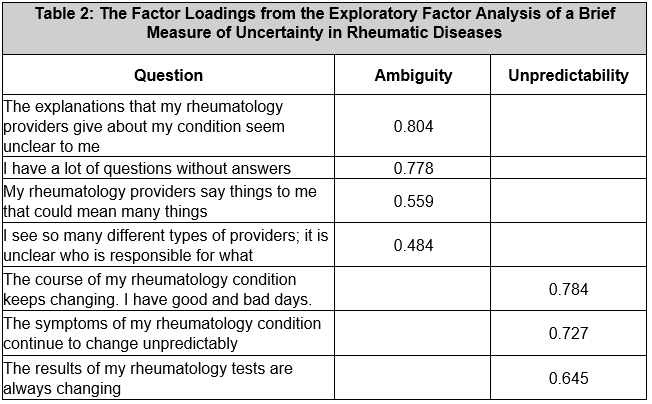
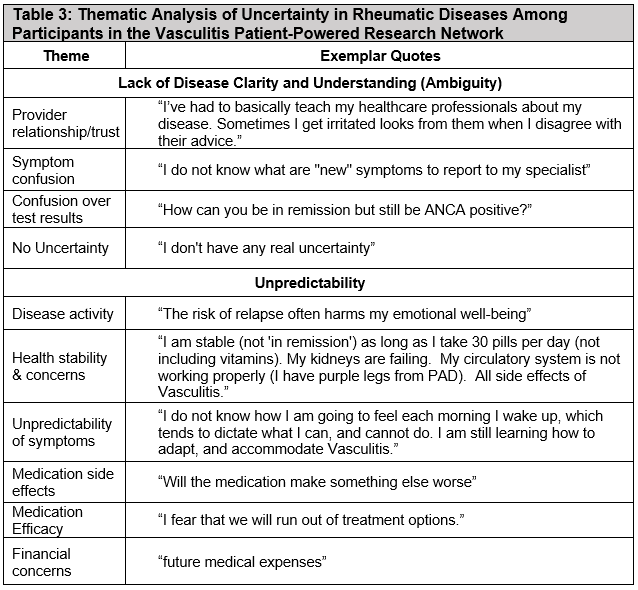
Results: The development cohort included 132 (31%) patients with AAV, 61 (46%) with IgG4-RD, and 30 (23%) with SSc (Table 1). The MUIS-S EFA retained seven of twenty-two original items. The brief measure included two factors: ambiguity and unpredictability (Table 2). There was high internal consistency for the brief measure (α=0.85) and its subscales: ambiguity (α=0.79) and unpredictability (α=0.81). Convergent validity of the full scale was found with anxiety (r=0.42, p< 0.001), depression (r=0.47, p< 0.001), sickness impact (r=0.34, p< 0.001), and sleep duration (r= -0.243, p< 0.001). Correlations between uncertainty and each of these distress metrics remained similar when adjusting for age and gender (all p< 0.001). For the CFA, we included 475 patients with vasculitis (Table 1, 294 [62%] AAV) and confirmed that the brief measure had good fit by multiple measures. Common sources of uncertainty cited in qualitative responses included disease activity, health stability and concerns, unpredictability of symptoms, provider relationship/trust, and symptom confusion (Table 3).
Conclusion: We developed a brief measure of uncertainty in rheumatic diseases with high internal consistency and convergent validity regarding measures of anxiety, depression, sickness impact, and sleep duration. This measure may be useful for research to develop interventions aimed at improving resiliency in the face of uncertainty, and in clinical practice to identify patients experiencing uncertainty.
To cite this abstract in AMA style:
Bolden C, Cook C, Finkelstein-Fox L, Fu X, Castelino F, Choi H, Perugino C, Stone J, Park E, Yeung C, Stone-Johnson C, Ivits S, Merkel P, Hall D, Wallace Z. Development and Validation of a Brief Measure of Uncertainty Among Patients with Vasculitis and Other Rheumatic Diseases [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/development-and-validation-of-a-brief-measure-of-uncertainty-among-patients-with-vasculitis-and-other-rheumatic-diseases/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-and-validation-of-a-brief-measure-of-uncertainty-among-patients-with-vasculitis-and-other-rheumatic-diseases/