Session Information
Date: Sunday, November 17, 2024
Title: Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Interstitial lung disease (ILD) and other pulmonary manifestations are a major cause of mortality and morbidity in patients with dermatomyositis (DM). The ProDERM study was a randomized Phase 3 study designed to evaluate the efficacy and safety of intravenous immunoglobulin (IVIg) in adult DM patients. Here, we performed a post-hoc analysis of ProDERM to assess the effect of IVIg on pulmonary symptoms of DM.
Methods: ProDERM was a placebo-controlled, double-blind, multicenter Phase 3 study involving two phases. From Weeks 0 to 16, patients were randomized to receive 2.0 g/kg IVIg or placebo (saline) every 4 weeks. This was followed by an open-label extension period from Weeks 16 to 40, during which all eligible patients received IVIg for six additional cycles. Pulmonary symptoms of DM were assessed by the physician and reported using the Myositis Disease Activity Assessment Tool (MDAAT). Visual analog scales (VAS) were scored over 10 cm to quantify overall pulmonary disease activity, with higher score indicating worse disease. Physicians also reported presence/absence of pulmonary symptoms of dysphonia, dyspnea or cough from active reversible ILD, and dyspnea from respiratory muscle weakness on MDAAT.
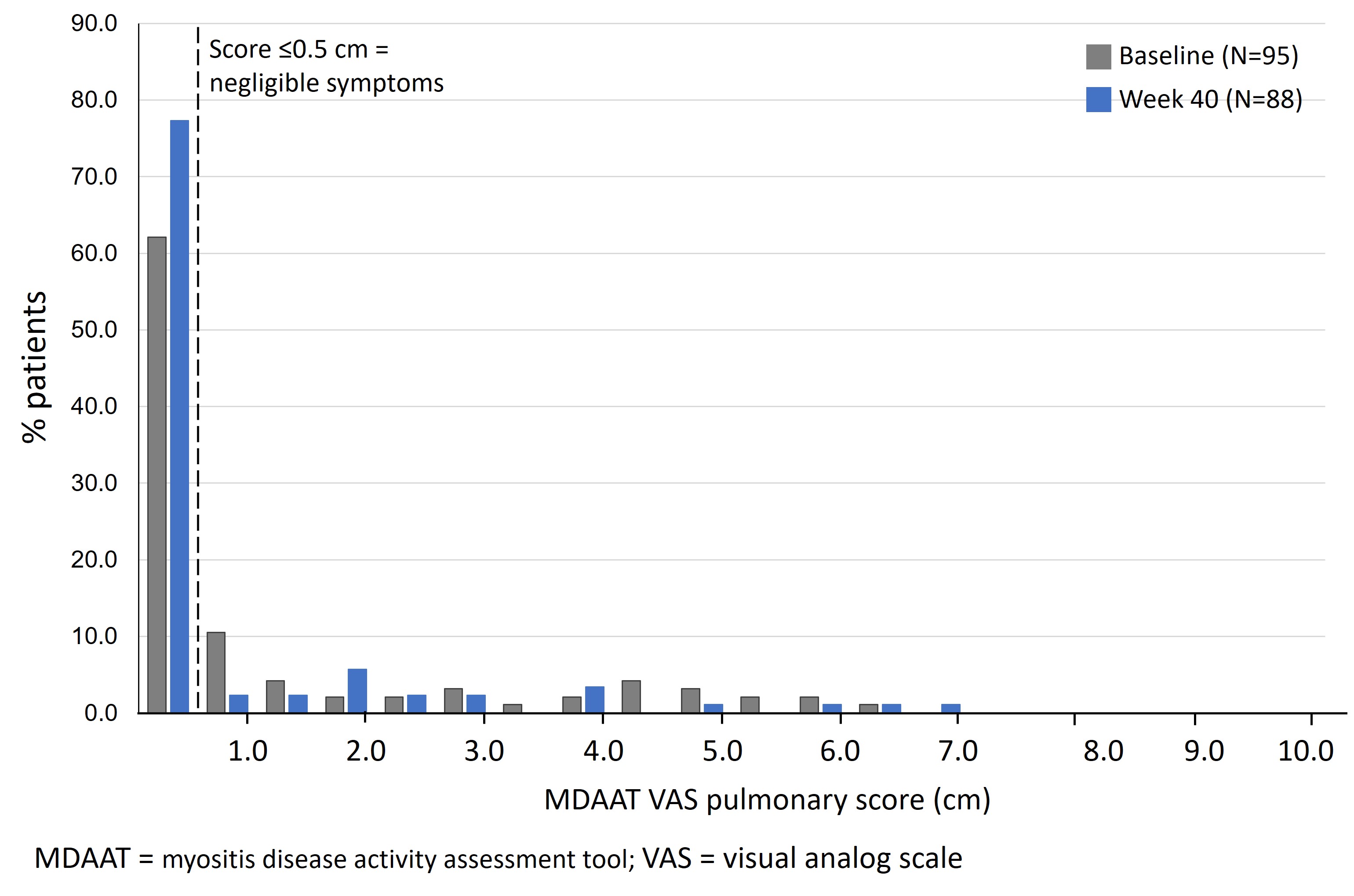
Results: Of 95 patients with DM in the ProDERM study, 98% met the probable or definite criteria for inflammatory myopathy according to the 2017 European League Against Rheumatism (EULAR)/American College of Rheumatology (ACR) criteria for idiopathic inflammatory myopathies. Of all patients, 59 (62.1%) had baseline pulmonary VAS ≤0.5 cm (Figure 1), indicating no myositis lung involvement. By Week 40, this increased to 68 of the 88 patients with available data (77.3%; p=0.007).
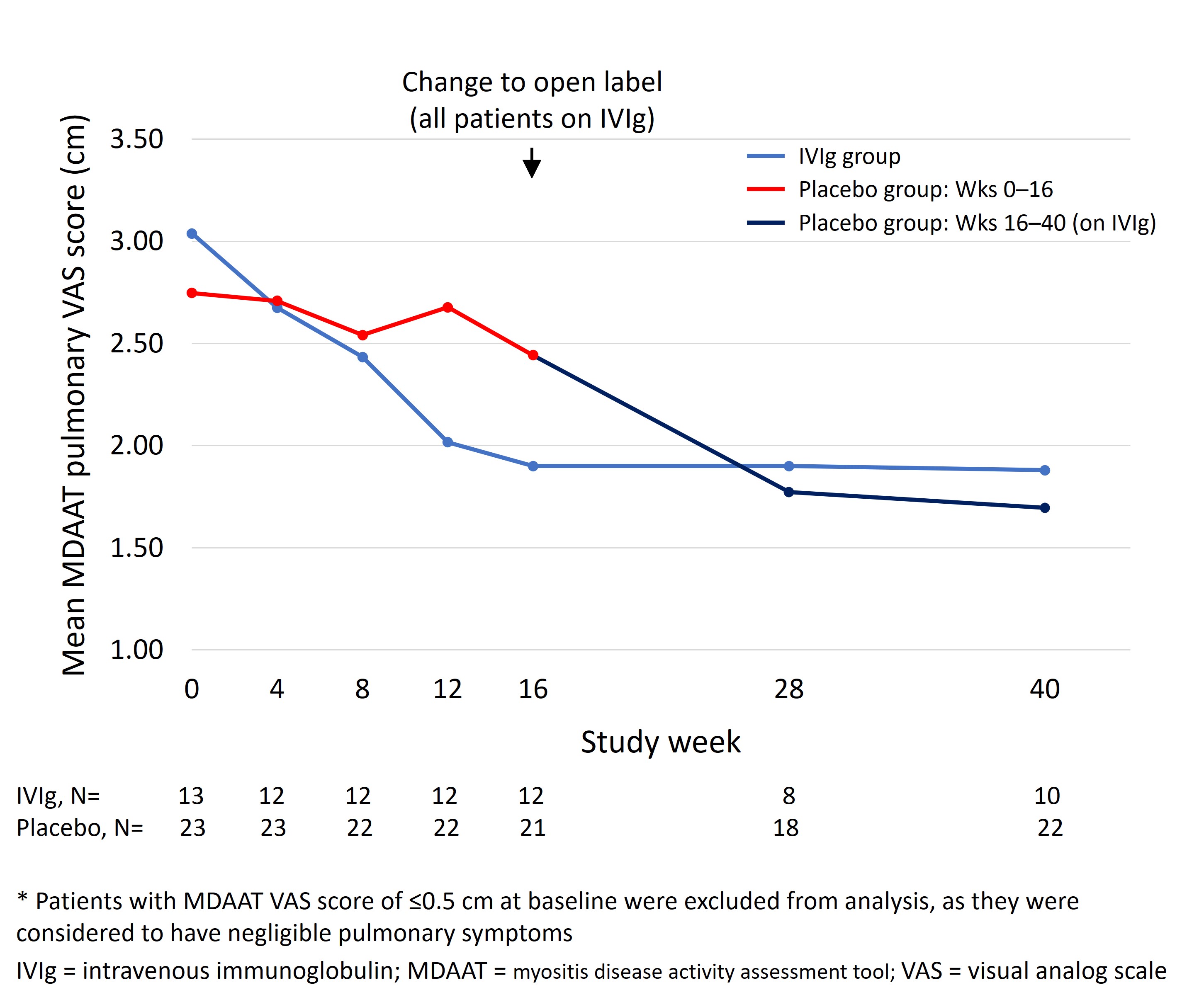
As patients with baseline pulmonary VAS ≤0.5 cm were unlikely to improve in terms of pulmonary manifestations during the trial, improvement in pulmonary score was examined only in the 36 patients with baseline VAS >0.5 cm (Figure 2). For patients with data at both baseline and Week 16, those on IVIg (n=12) experienced a mean decrease of 1.15 cm (37.7%; p=0.001) vs. 0.17 cm (6.5%) in patients on placebo (n=21; p=0.50). From baseline to Week 40, with patients on placebo having switched to IVIg, the mean decrease for the combined cohort (n=32 patients with data) was 1.08 cm (38.2%; p< 0.001).
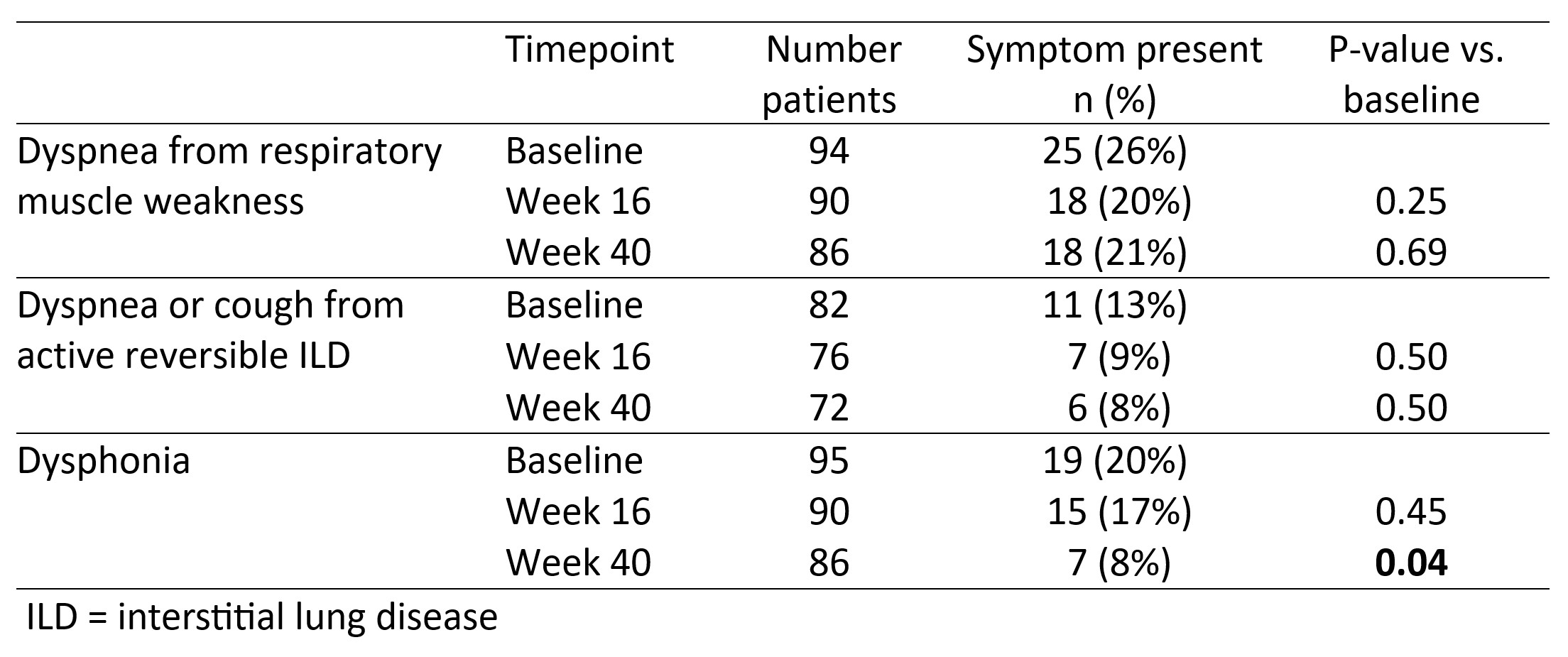
Among all patients, the percentage with dyspnea from respiratory muscle weakness, or from ILD, both decreased from baseline numerically at Weeks 16 and 40, but neither reached statistical significance (Table 1). The percentage of patients with dysphonia decreased from 20% at baseline to 8% at Week 40 (p=0.04).
Conclusion: IVIg may have favorable treatment effects on active pulmonary manifestations of DM. Further studies are warranted.
To cite this abstract in AMA style:
Aggarwal R, Charles-Schoeman c, Schessl J. Efficacy of Intravenous Immunoglobulin (Octagam 10%) on Pulmonary Manifestations in Patients with Dermatomyositis: Results from the ProDERM Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/efficacy-of-intravenous-immunoglobulin-octagam-10-on-pulmonary-manifestations-in-patients-with-dermatomyositis-results-from-the-proderm-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-intravenous-immunoglobulin-octagam-10-on-pulmonary-manifestations-in-patients-with-dermatomyositis-results-from-the-proderm-study/