Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: The primary objective was to investigate subjects with rheumatic disease (Systemic Lupus Erythematosus (SLE), Sjogrens disease (SjD), and Rheumatoid Arthritis (RA)) who initiated HCQ treatment and manually interpret QTc interval changes following 12 weeks of treatment in relation to whole blood HCQ levels. The secondary objective was to assess if other QTc prolonging medications or anti-SSA autoantibody positivity were associated with the increased risk for QTc prolongation.
Methods: A cohort of subjects meeting the ACR/EULAR criteria for SLE, RA, or SjD who were newly prescribed HCQ were enrolled in this 12-week prospective observational study. Subjects underwent EKGs at weeks 1 and 12, with a subset of patients receiving access to the AliveCor mobile application and KardiaMobile 6L device for weekly, at home, monitoring. Real-time QTc interval assessment was conducted using the AliveCor app, utilizing an FDA-cleared algorithm developed by GE Healthcare. Whole blood HCQ levels were measured at the end of study period for each subject. Wilcoxon Rank Sum tests were used to evaluate anti-SSA autoantibody (Ro) positivity and change in QTc. Pearson correlation and paired t-test were used to evaluate HCQ levels and change in QTc. QTc measurements were manually measured and calculated using the Bazett formula.
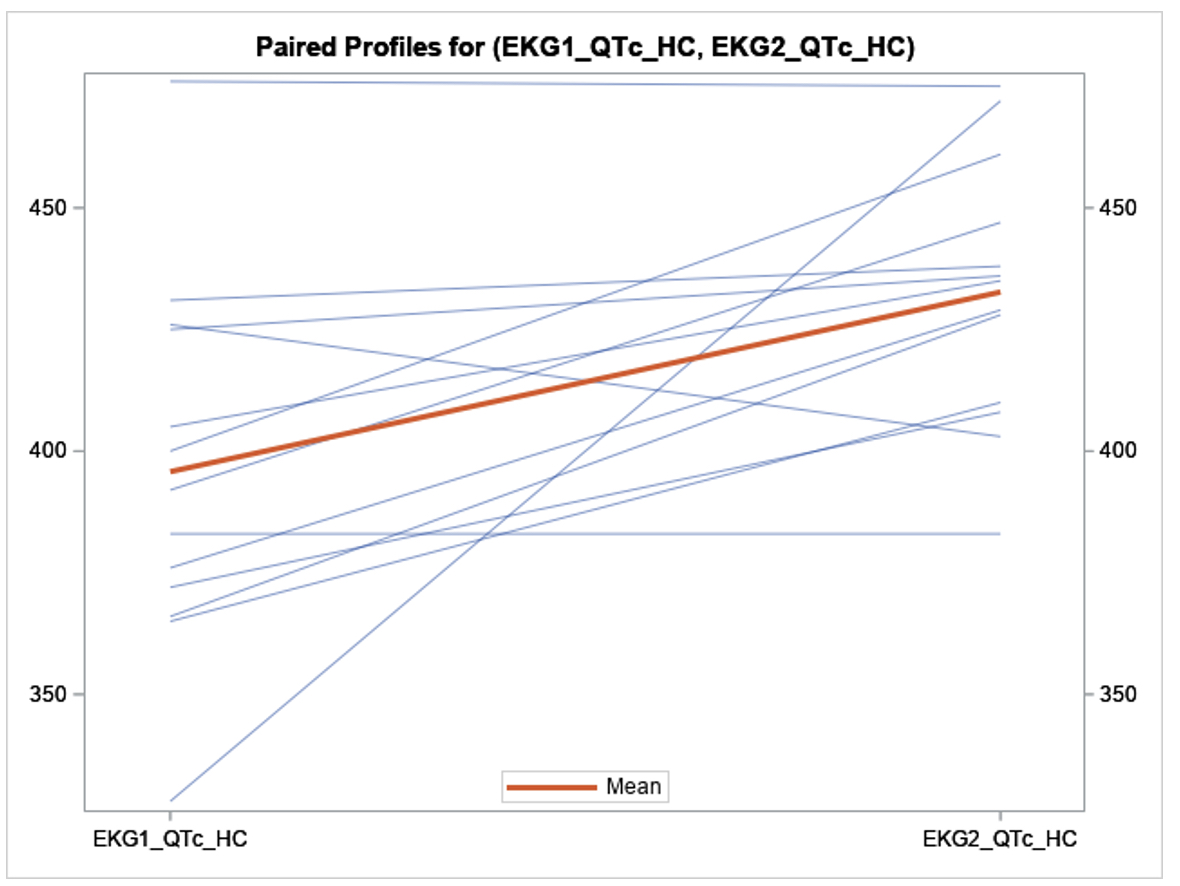
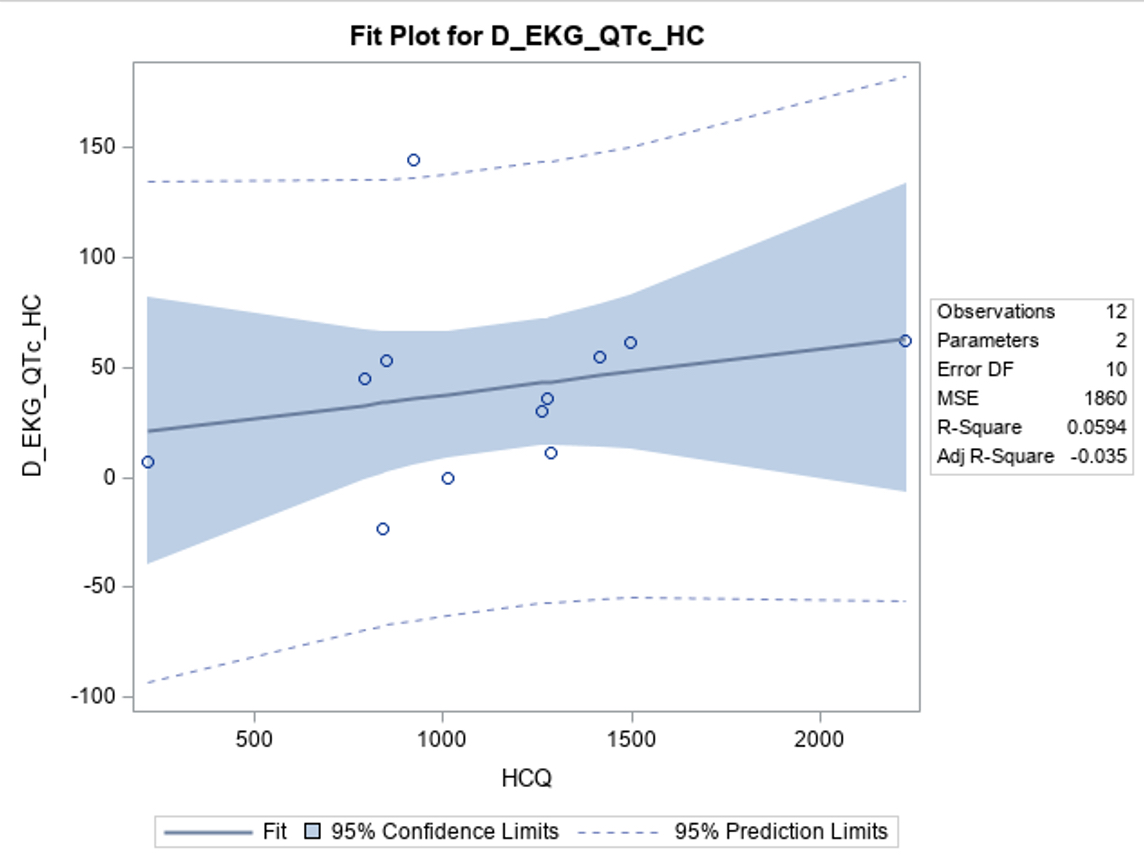
Results: Of the 14 patients to complete the study period, 13 had HCQ levels resulted by June 17
th, 2024, and 11 completed 12 weeks of Kardia monitoring. The mean age was 46.9 years old; Range 26 – 76), 1 patient was male, 6 patients were SSA positive, 4 patients were on other QTc prolonging medications, 2 patients had CKD, and no patients had cardiovascular disease. The mean HCQ blood levels were 1192.07 ng/mL, standard deviation (SD) 516.4 at 12 weeks of treatment. Notably, 12 weeks following HCQ initiation, subjects exhibited a mean QTc interval increase on 12-lead EKG of 36.9 ms (95% CI: 11.4 – 62.4; SD 42.2, p = 0.008) on paired t-test analysis as compared to baseline. This increase persisted in subjects without chronic kidney disease (CKD) or concurrent use of other QT-prolonging medications. Notably, a statistically insignificant correlation was observed between HCQ levels and the change in QTc interval (R-Square = 0.06, p = 0.45). Subjects who underwent the KardiaMobile remote monitoring were not found to have an increase in QTc from baseline. There was no statistically significant correlation between SSA autoantibodies status and change in QTc. No subjects suffered adverse cardiac events.
Conclusion: Our preliminary findings indicate a statistically significant increase in QTc. The observed weak correlation between whole blood HCQ levels and QTc changes suggests a larger sample size is needed to determine its significance. While prior studies have proposed a time-dependent relationship with the maximal cardiac effects of HCQ, our findings may suggest an earlier onset of QTc prolongation of 12 weeks. As the study continues, further data will be essential to provide deeper insights into these relationships.
To cite this abstract in AMA style:
Azzam J, Fares J, Pusapati n, Venuturupalli S, Wallace D. Rheumatic Disease Patients: Assessment of Hydroxychloroquine’s Effects on QTc Intervals with Weight Based Dosing (RAISE-QT) Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/rheumatic-disease-patients-assessment-of-hydroxychloroquines-effects-on-qtc-intervals-with-weight-based-dosing-raise-qt-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rheumatic-disease-patients-assessment-of-hydroxychloroquines-effects-on-qtc-intervals-with-weight-based-dosing-raise-qt-study/