Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Tumor necrosis factor inhibitors (TNFi) are generally the first class of biologic disease modifying anti-rheumatic drugs (DMARDs) prescribed for rheumatoid arthritis (RA) patients with an inadequate response to conventional synthetic DMARDs. A molecular signal response classifier (MSRC) predicting inadequate response to TNFi was developed, validated and shown to improve patient outcomes. Studies in RA testing the utility of MSRC have been conducted with white, non-Hispanic patient samples. Data on disease response to medications for other ethnic groups is sparse. Hispanic patients are more frequently under/ uninsured and encounter barriers to health care leading to worse outcomes. We here evaluated the performance of MSRC in a majority Hispanic, low socioeconomic status sample of RA patients with at least moderate disease activity.
Methods: Sixty-one participants from a single center were identified via retrospective chart review. All screened patients fulfilled 2010 classification criteria for RA and had at least moderate disease activity measured as clinical disease activity index (CDAI) >10 at entry. All were tested with MSRC and had a decision on bDMARD choice made after receiving the MSRC test result. The outcome was achievement of CDAI low disease activity or remission (CDAI-LDA/REM).
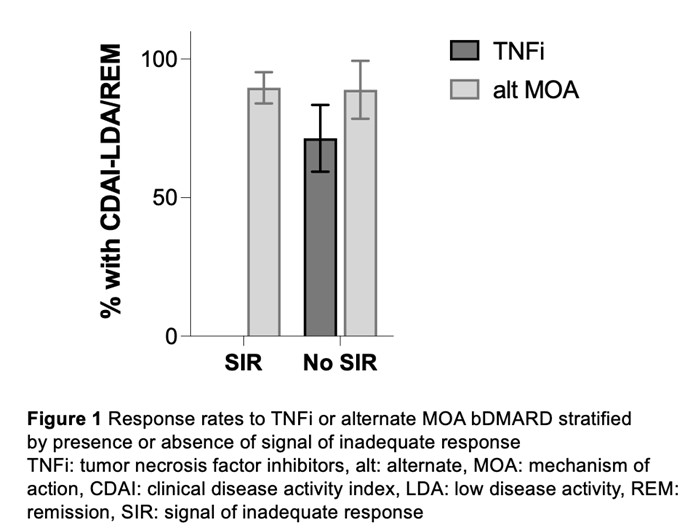
Results: Among 61 tested patients, three were excluded due to missing CDAI at follow-up and five due to no advancement to bDMARD treatment. In the final sample (N=53), mean < ![if !msEquation] >< ![if !vml] >![]() < ![endif] >< ![endif] >standard deviation age was 53.7±12.6, 40/53 (75%) were female, 49/53 (93%) were Hispanic, 49/53 (93%) were anticitrullinated protein antibody positive, 24/53 (45%) had prior TNF exposure, and mean baseline CDAI was 28.0±11.0. Of 53 analyzed patients 30/53 (56.6%) received a signal of inadequate response (SIR) to TNFi and 23/53, (43.4%) exhibited no SIR. Twenty-nine patients with SIR received treatment with an alternate mechanism of action (MOA) and 26/29 (89.7%) achieved CDAI-LDA/REM over an average of 4.48±1.86 months (Figure 1). One patient with SIR received TNFi and did not achieve CDAI-LDA/REM. Of 23 patients without SIR 14/23 (60.9%) were prescribed TNFi and 12/14 had no prior TNFi exposure; 10/14 (71.4%) achieved CDAI-LDA/REM over 3.5±0.9 months. The remaining 9/23 (39.1%) were prescribed an alternate MOA bDMARD and 2/9 had no prior TNFi exposure; 8/9 (88.9%) attained CDAI-LDA/REM over 3.2±0.7 months (p-for-difference=0.322). The difference in response to TNFi versus alternate MOA approaches among patients without SIR remained non-significant after adjusting for anticitrullinated protein antibody status and prior TNFi exposure.
< ![endif] >< ![endif] >standard deviation age was 53.7±12.6, 40/53 (75%) were female, 49/53 (93%) were Hispanic, 49/53 (93%) were anticitrullinated protein antibody positive, 24/53 (45%) had prior TNF exposure, and mean baseline CDAI was 28.0±11.0. Of 53 analyzed patients 30/53 (56.6%) received a signal of inadequate response (SIR) to TNFi and 23/53, (43.4%) exhibited no SIR. Twenty-nine patients with SIR received treatment with an alternate mechanism of action (MOA) and 26/29 (89.7%) achieved CDAI-LDA/REM over an average of 4.48±1.86 months (Figure 1). One patient with SIR received TNFi and did not achieve CDAI-LDA/REM. Of 23 patients without SIR 14/23 (60.9%) were prescribed TNFi and 12/14 had no prior TNFi exposure; 10/14 (71.4%) achieved CDAI-LDA/REM over 3.5±0.9 months. The remaining 9/23 (39.1%) were prescribed an alternate MOA bDMARD and 2/9 had no prior TNFi exposure; 8/9 (88.9%) attained CDAI-LDA/REM over 3.2±0.7 months (p-for-difference=0.322). The difference in response to TNFi versus alternate MOA approaches among patients without SIR remained non-significant after adjusting for anticitrullinated protein antibody status and prior TNFi exposure.
Conclusion: The rate of SIR to TNFi in this sample of Hispanic patients with RA is similar to the 56.4% observed in a larger study of non-Hispanic white patients. MSRC impacted bDMARD choice when an SIR was present and in patients without SIR and no prior TNFi exposure. Compared to a typical 25-40% attainment of CDAI-LDA at 12 months in response to any arbitrary biologic choice, treatment selection informed by MSRC resulted in superior rates of attainment of therapeutic target.
To cite this abstract in AMA style:
Bui V, Rodriguez M, Ormseth S. Performance of a Molecular Signal Response Classifier Predicting Inadequate Response to Tumor Necrosis Factor Inhibitors in Hispanic Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/performance-of-a-molecular-signal-response-classifier-predicting-inadequate-response-to-tumor-necrosis-factor-inhibitors-in-hispanic-patients-with-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/performance-of-a-molecular-signal-response-classifier-predicting-inadequate-response-to-tumor-necrosis-factor-inhibitors-in-hispanic-patients-with-rheumatoid-arthritis/