Session Information
Date: Saturday, November 16, 2024
Title: Abstracts: SLE – Diagnosis, Manifestations, & Outcomes I: Omics
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: SLE is a leading cause of premature death, often from severe complications including lupus nephritis (LN), especially in disadvantaged groups. Despite advances in treatment, 10-30% of patients with LN progress to end-stage kidney disease. While disease-associated dysbiosis within gut microbiota has been reported, causative roles remain poorly understood. We found ~40% of LN flares are associated with gut blooms of Ruminococcus gnavus (RG) strains expressing a novel cell wall lipoglycan (LG)1,2,3. We sought to define underlying in vivo pathogenic pathways.
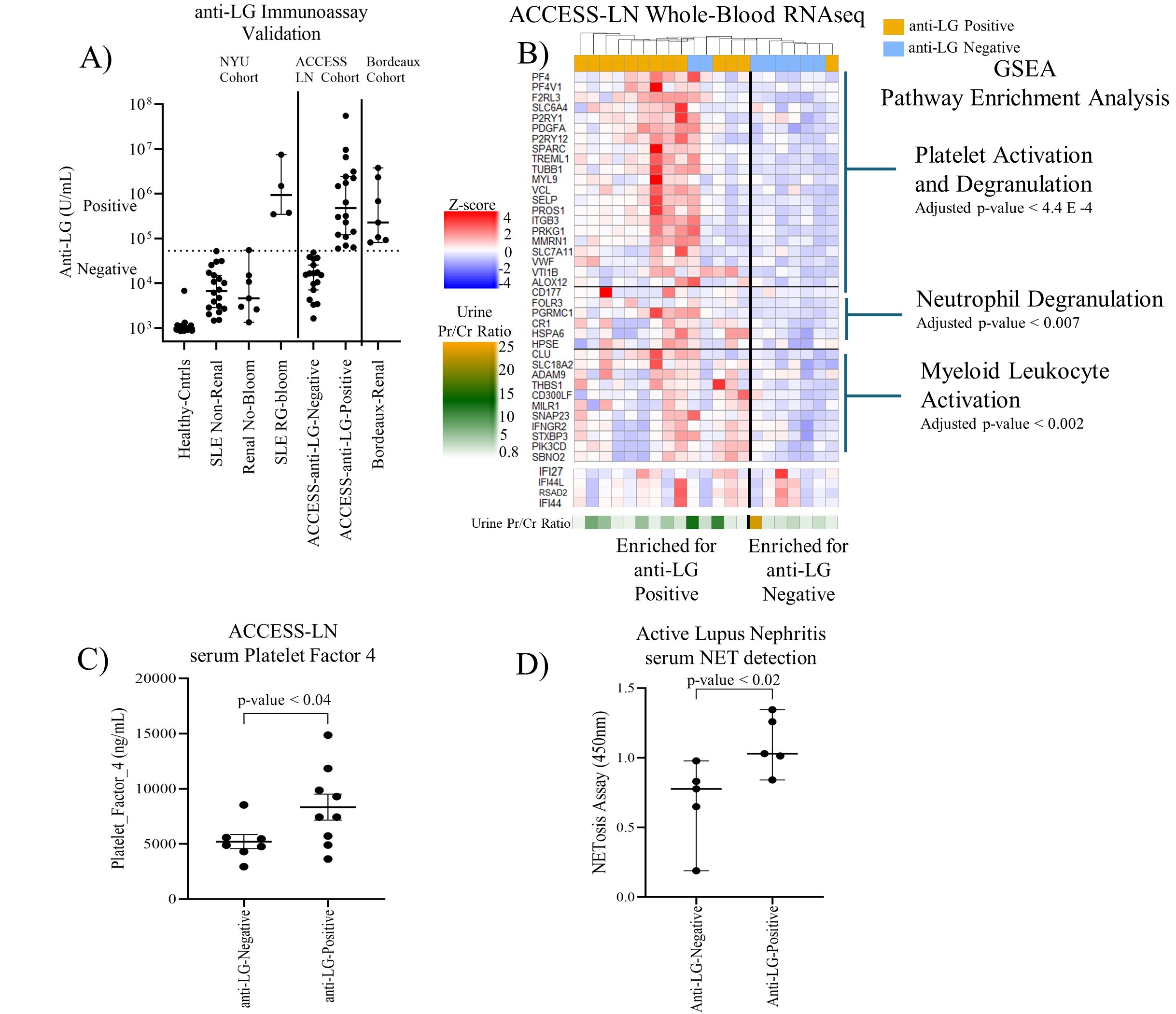
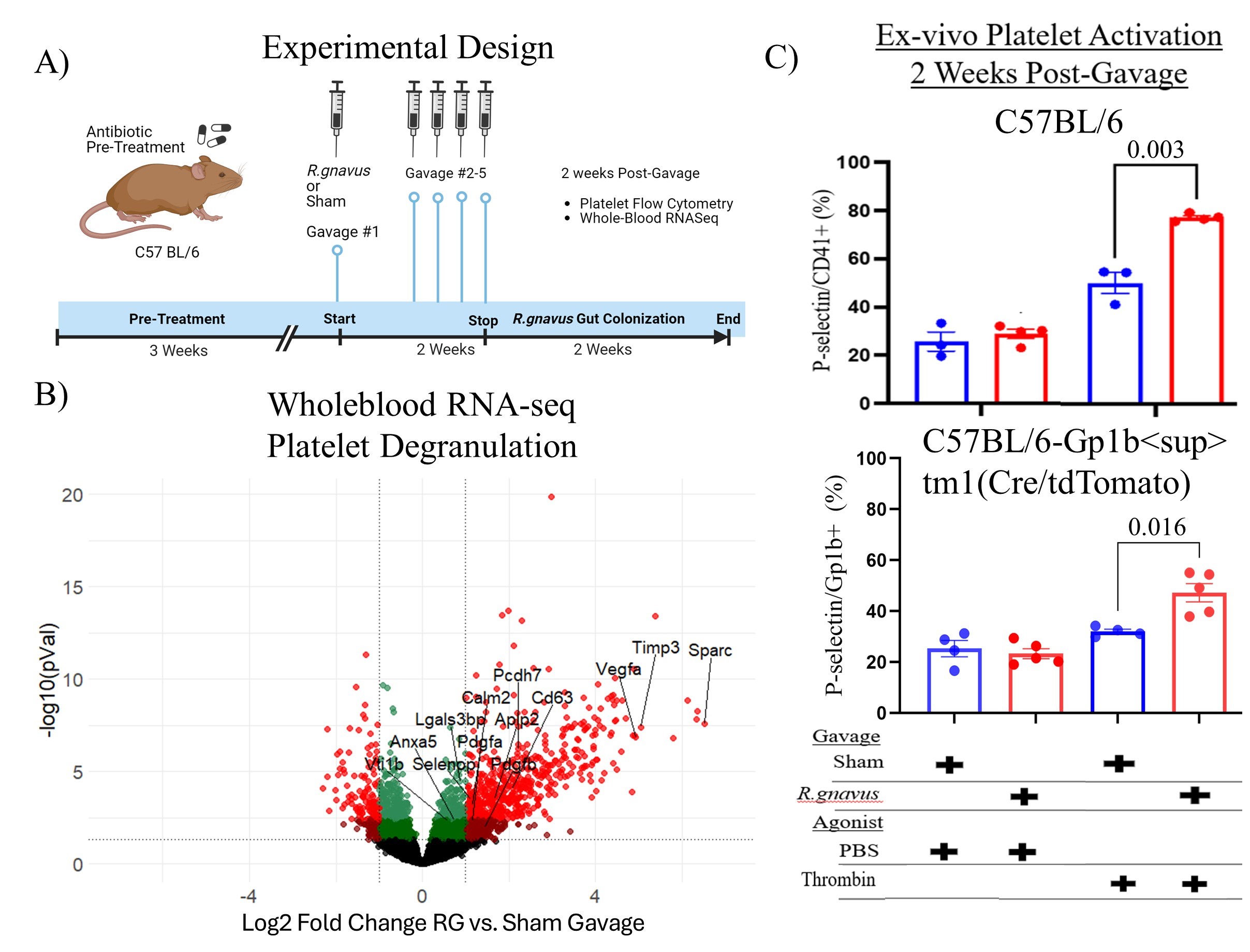
Methods: Patients in three LN cohorts, NYU, and the validation cohorts: Bordeaux Hospital University and ITN_ACCESS_LN, were investigated with a quantitative bead-based assay for serum anti-LG IgG (Fig1A). Bulk whole-blood RNA-seq libraries were analyzed for gene and GSEA pathway representation. PF4/CXCL4 levels were assessed by ELISA and platelet-derived microparticles (MPs) by microfluorimetry. NETosis was measured via immunoassays for serum citrullinated histone-H3, DNA, and myeloperoxidase. To assess causation, naïve C57BL/6 female mice were gut-colonized with an LG-producing RG strain, and then whole-blood RNA-seq and flow cytometry were performed.
Results: In LN validation cohort patients with high anti-LG levels, whole-blood RNAseq revealed increased signatures for platelet activation and degranulation (qval< 4.4E-4), neutrophil degranulation (qval< 0.007), and myeloid leukocyte activation (qval< 0.002), independent of type I IFN signatures (Fig1B). Higher levels of PF4, a platelet granule protein, were associated with high anti-LG levels (p< 0.04)(Fig1C) and significantly higher NETosis fragments were also detectable in anti-LG positive LN patients (p< 0.02, Fig1D). Active-LN patients from the Bordeaux Hospital University cohort showed a positive trend between anti-LG levels and P-selectin+ platelet MPs. In these studies, anti-dsDNA levels did not correlate with platelet activation or NETosis. In mice colonized with LG-producing RG (Fig2A), blood-bulk RNAseq showed an increased platelet degranulation gene signature (p< 0.002, Fig2B). Notably, SPARC, PDGFA, and VTI1B were significantly increased in RG-colonized mice (p< 0.05), just as in LN patients. RG-colonization induced ex vivo platelet sensitization after thrombin challenge, detected as raised surface P-selectin levels (p<0.016)(Fig2C).
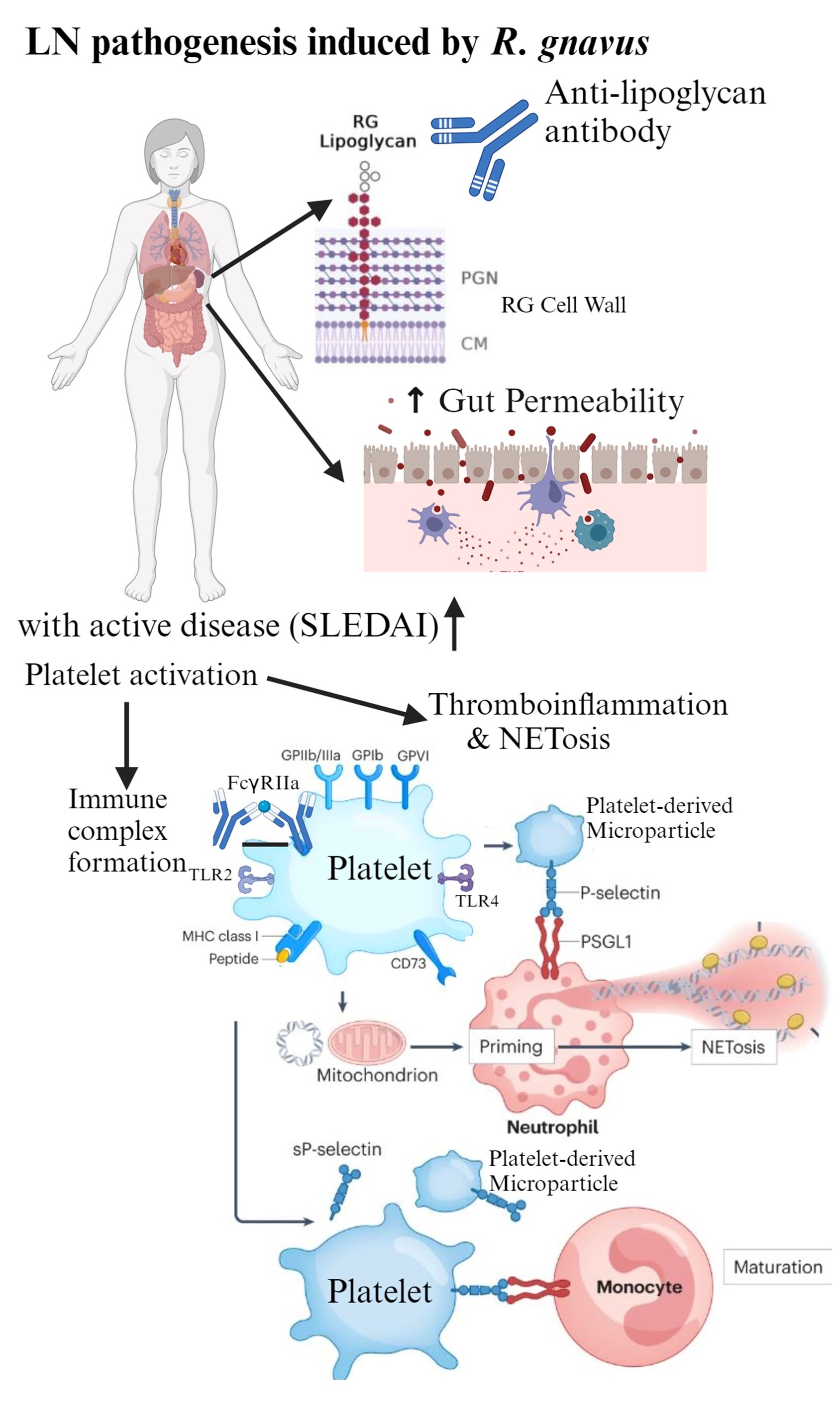
Conclusion: Our findings provide the first evidence that RG intestinal blooms trigger LN flares linked to platelet activation with MP formation and downstream NETosis (Fig3), in a type-I IFN-independent setting. Using the anti-LG antibody as a companion diagnostic, this set of active LN patients should be evaluated for therapeutic interventions of platelet activation and NETosis.
References:
1. Azzouz, D et al. ARD 2019
2. Azzouz, D et al. ARD 2023
3. Silverman, GJ et al. NRR 2023
To cite this abstract in AMA style:
Amarnani A, Rivera-Martinez C, Scherlinger M, Azzouz D, Lee A, Trujillo K, Cornwell M, Weinstein T, Borja T, Matta B, Chung S, Cooney L, Chaudhary U, Medvedovsky S, Izmirly P, Buyon J, Blanco P, Barnes B, Ramkhelawon B, Ruggles K, Silverman G. A Human Gut Pathobiont Drives Platelet Activation with Microparticle Release and NETosis During Lupus Nephritis Flares [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/a-human-gut-pathobiont-drives-platelet-activation-with-microparticle-release-and-netosis-during-lupus-nephritis-flares/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-human-gut-pathobiont-drives-platelet-activation-with-microparticle-release-and-netosis-during-lupus-nephritis-flares/