Session Information
Date: Saturday, November 16, 2024
Title: SLE – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Systemic lupus erythematosus (SLE) is a heterogeneous autoimmune disease with diverse clinical manifestations and organ involvement, posing significant challenges in its management. The emergence of biologics has increased therapeutic efficacy and reduced the long-term adverse events associated with glucocorticoid use. Although randomized, double-blind, placebo-controlled trials have demonstrated the efficacy of belimumab and anifrolumab as add-on therapies to standard treatment, there is currently a lack of evidence regarding the optimal timing for the introduction of biologic agents in SLE management. Consequently, determining how to effectively incorporate these biologic agents into SLE treatment is crucial. This study aimed to investigate the optimal timing for the introduction of biologic agents in SLE treatment.
Methods: The PLEASURE-J study is a multicenter, prospective cohort study involving 127 institutions in Japan, focusing on early-onset SLE patients aged 40 years or younger at the time of diagnosis. Data were extracted from the PLEAJURE J cohort, identifying patients who received biologic agents (belimumab, anifrolumab, or rituximab). These patients were divided into two groups based on the timing of treatment introduction: early (within 6 months) or maintenance phase (after 6 months). The observation period was set at 3 years, and relapse was defined as an increase in prednisolone (PSL) to 4 mg or more, at the discretion of the attending physician. Outcomes (PSL dose, relapse rates, number of relapses, complications, disease activity) between the two groups were compared.
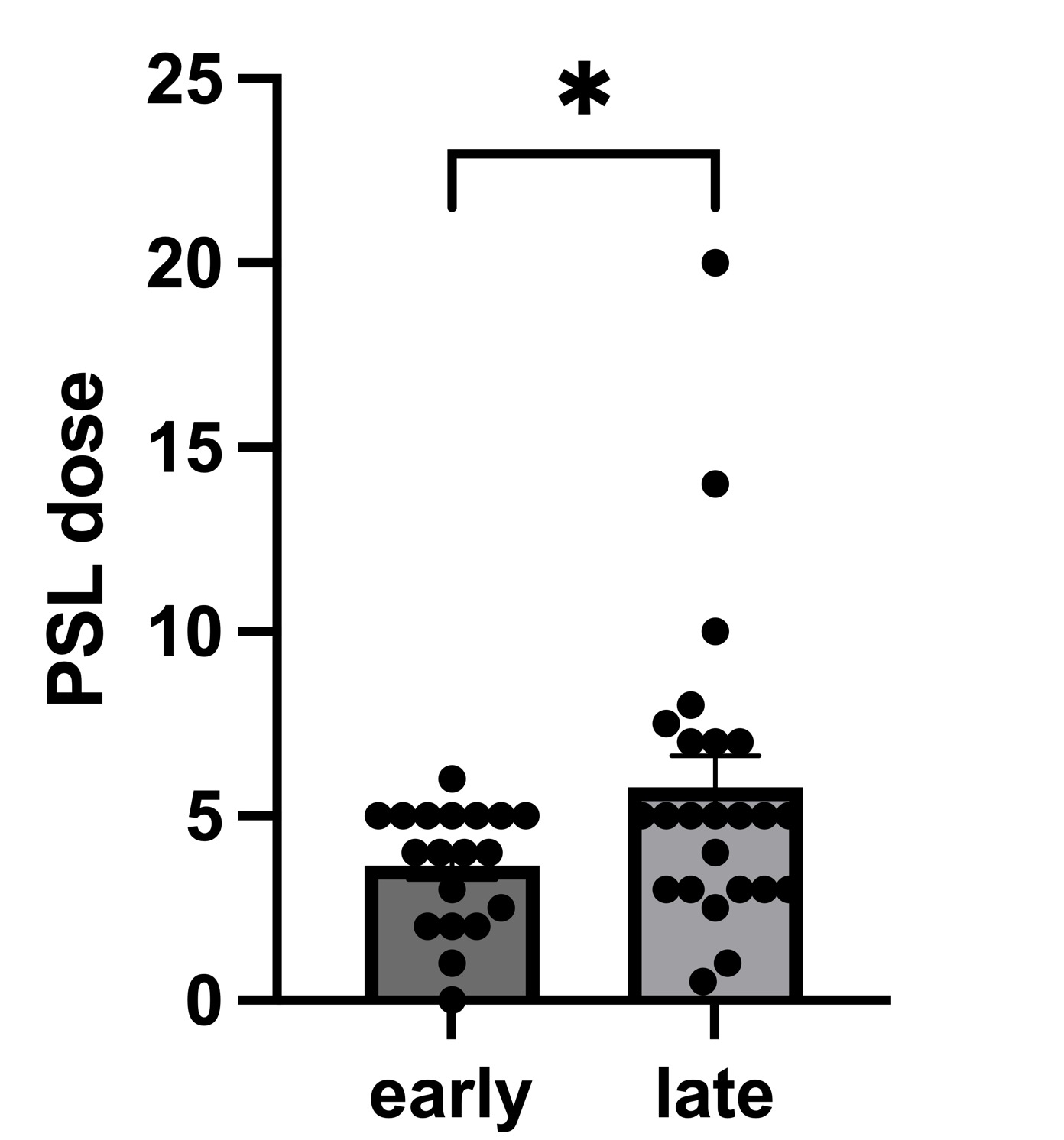
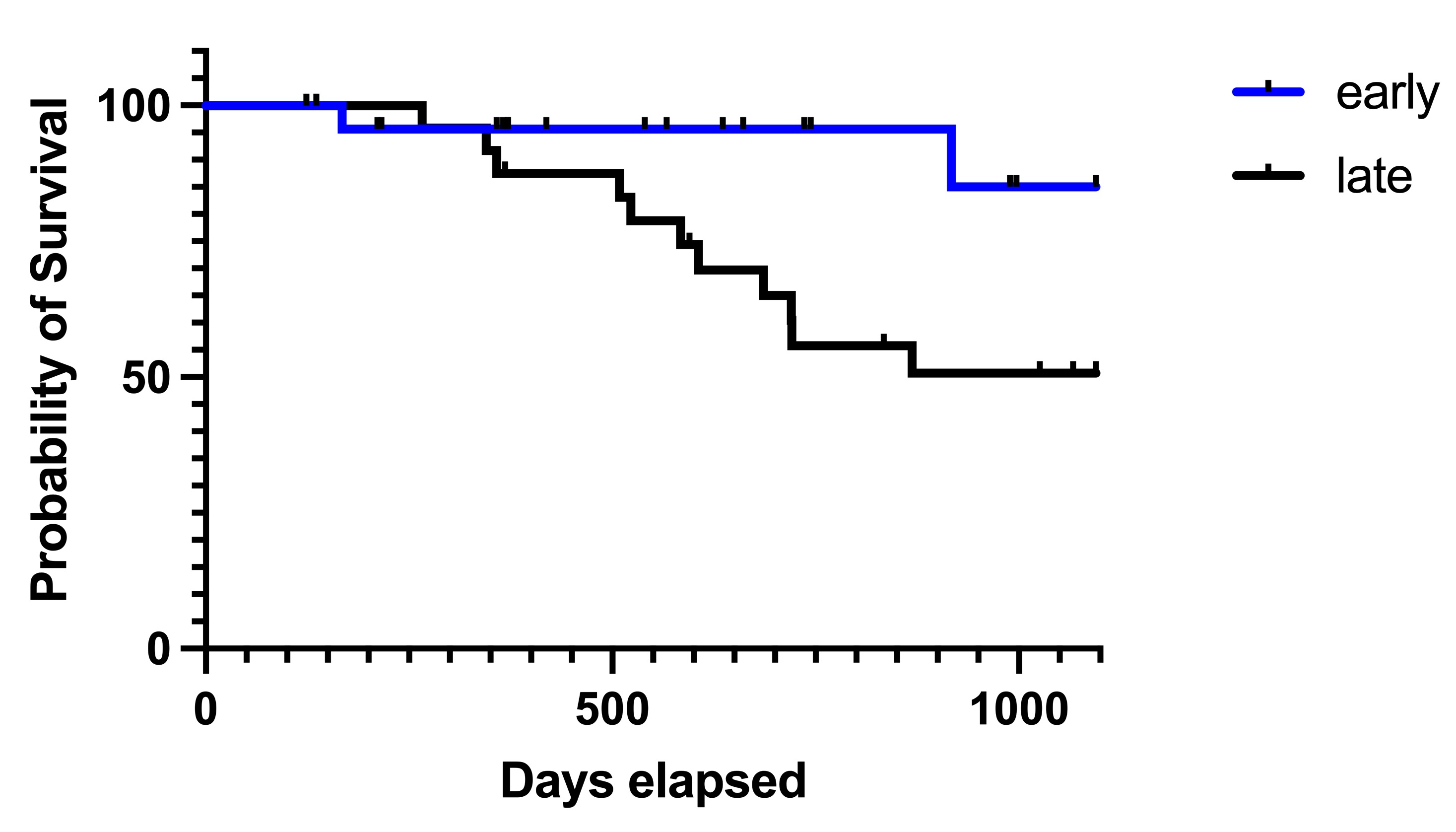
Results: Patients who received biologic agents (belimumab, anifrolumab, or rituximab; n=45, 5, and 3, respectively) were identified. The group that received early introduction of biologic agents required a lower maintenance PSL dose compared to the group that received biologic agents in the maintenance phase (mean of early group: 3.658 mg [95% confidence interval:2.862-4.453], mean of late group: 5.771 mg [3.993-7.548], p=0.0456) (Figure 1). Additionally, the early introduction group experienced a significant reduction in relapse rates [Log-rank test, p=0.0310] (Figure 2).
Conclusion: Early introduction of biologic agents in SLE not only suppresses disease relapse but also enables a reduction in the maintenance PSL dose. The early introduction of biologic agents may positively impact long-term outcomes over a period of 3 years.
To cite this abstract in AMA style:
HIrayama T, Tsujimoto K, Kaneko K, Isojima S, Ichinose K, Iwata Y, Oku K, Sada K, Tanaka Y, Nakajima A, Fujio K, Matsushita M, Miyamae T, Murashima A, Kumanogoh A. Early Introduction of Biologic Agents in Systemic Lupus Erythematosus Reduces Relapse and Glucocorticoid Maintenance Dose: A Cohort Study from the PLEAJURE J Registry [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/early-introduction-of-biologic-agents-in-systemic-lupus-erythematosus-reduces-relapse-and-glucocorticoid-maintenance-dose-a-cohort-study-from-the-pleajure-j-registry/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/early-introduction-of-biologic-agents-in-systemic-lupus-erythematosus-reduces-relapse-and-glucocorticoid-maintenance-dose-a-cohort-study-from-the-pleajure-j-registry/