Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Psoriatic arthritis (PsA) is associated with metabolic and cardiovascular disease. Studies have suggested that treatment with apremilast is associated with weight loss and other cardio-metabolic benefits. This study aimed to examine the effects of apremilast on body weight and composition and cardiovascular risk factors in psoriatic arthritis patients.
Methods: Consecutive adults with active PsA initiating apremilast (30 mg twice daily after a step-up regime) were included. Abdominal fat was assessed using Dual Energy X-ray Absorptiometry (DEXA) and disease activity was measured with DAS28-CRP at baseline, 26 and 52 weeks after initiation of apremilast. Data were analyzed using a linear mixed model. Body mass measures were adjusted for alcohol consumption, diarrhea, nausea, smoking, and physical activity.
Results: 44 patients were included, of whom 17 completed the study. Baseline characteristics; mean age 56 years (± 11 SD), median PsA duration five years [range 2–12], and a median BMI of 28 kg/m2 [range 24–33].
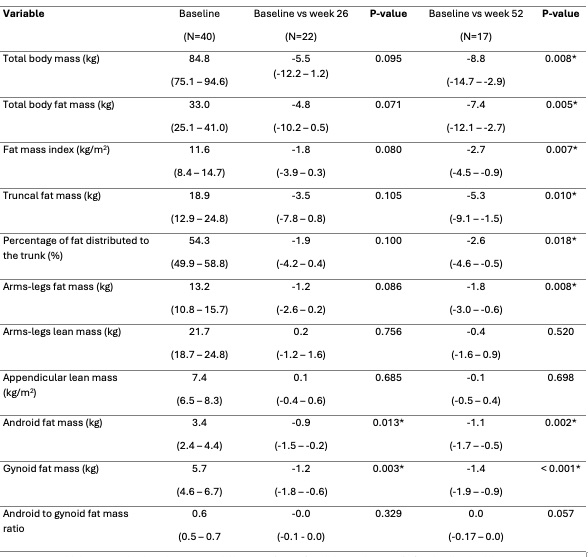
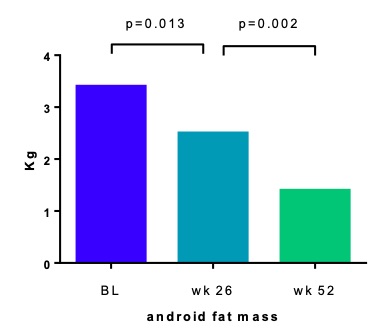
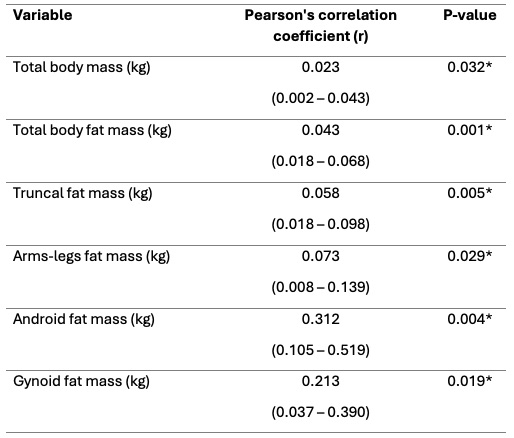
Following one year of treatment significant reductions were observed in multiple body mass parameters, including a decrease in android fat mass of 1.1 kg (95% CI [–1.7 – –0.5]; p=0.002). Correlation analysis revealed significant correlations between abdominal fat measures and a reduced disease activity, with the strongest correlation for android fat mass (0.309 (95% CI [0.101 – 0.517]; p=0.004). Additionally, in this cohort weight loss correlated with reduced disease activity.
Conclusion: This study demonstrates that apremilast reduces fat mass in PsA patients and indicates beneficial cardiovascular and metabolic effects, reducing the risk for cardiovascular events.
Values are depicted as mean difference (95% Confidence interval). Statically significant p-values (p < 0.05) are marked with *. Abbreviations: Kilogram (kg); Kilogram per square meter (kg/m2).
Mean android fat mass at baseline, week 26 and week 52.
Values are depicted as mean difference (95% Confidence interval). Statically significant p-values (p < 0.05) are marked with *. Abbreviations: Disease Activity Score_28 with c-reactive protein (DAS28-CRP); Kilogram (kg).
To cite this abstract in AMA style:
van Geel E, Hansildaar R, Çoban F, Dijkshoorn B, Heslinga M, Bos R, Korteweg M, Van Kuijk A, Nurmohamed M. Cardio-metabolic Effects of Apremilast in Patients with Psoriatic Arthritis: A Prospective Cohort Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/cardio-metabolic-effects-of-apremilast-in-patients-with-psoriatic-arthritis-a-prospective-cohort-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cardio-metabolic-effects-of-apremilast-in-patients-with-psoriatic-arthritis-a-prospective-cohort-study/