Session Information
Date: Saturday, November 16, 2024
Title: RA – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Individuals with rheumatoid arthritis (RA) may discontinue b/tsDMARDs due to treatment failure, adverse events or costs. Little is known about b/tsDMARDs discontinuation among Medicare beneficiaries with RA. The discontinuation profile to b/tsDMARDs may vary in RA given the different route of administration. We compared the discontinuation across b/tsDMARDs (including tumor necrosis factor inhibitors (TNFi bDMARDs), non-TNFi bDMARDs, and Janus kinase inhibitors (JAKi)).
Methods: This study used an observational, retrospective, new-user design utilizing 2012-2020 5% national Medicare claims data, including older (≥65) adults with RA based on the International Classification of Diseases, Ninth and Tenth Revision and initiating b/tsDMARDs (the first prescription is the index date) between 2013 and 2019. Patients had continuous Parts A, B and D but not Part C enrollment during the 12-month baseline period. The primary outcome was discontinuation, defined as having a medication gap ≥ 60 days. Censoring criteria included treatment switch, loss to follow up due to insurance disenrollment, end of study period or death. Demographics (age, sex, race, index year, low-income subsidy program enrollment, Medicare/Medicaid dual eligibility, region) and clinical characteristics (frailty score, RA severity index, Elixhauser comorbidity index, comorbidities, comedications and healthcare resource utilization) were assessed as baseline covariates influencing discontinuation. The association between time to discontinuation and type of b/tsDMARDs, adjusted for all covariates was evaluated using multivariable Cox proportional hazards (PH) regression model.
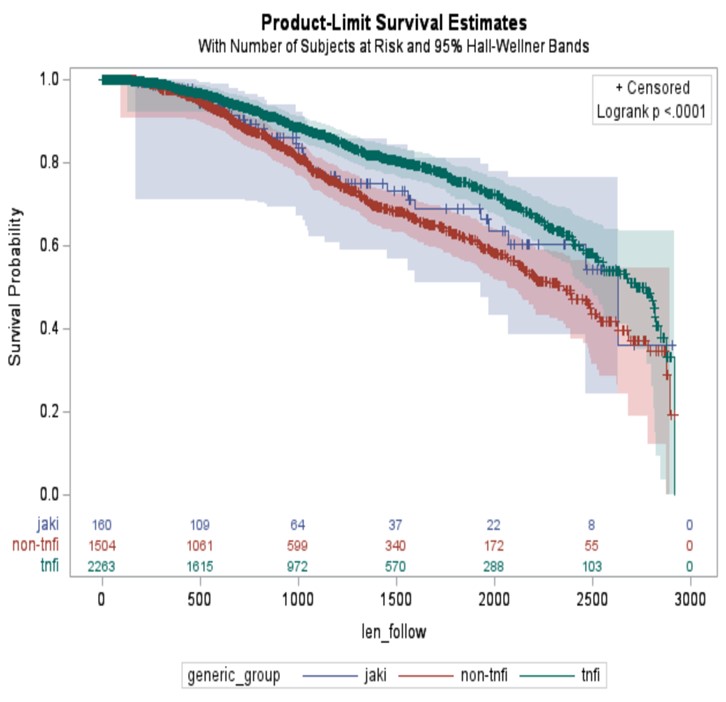
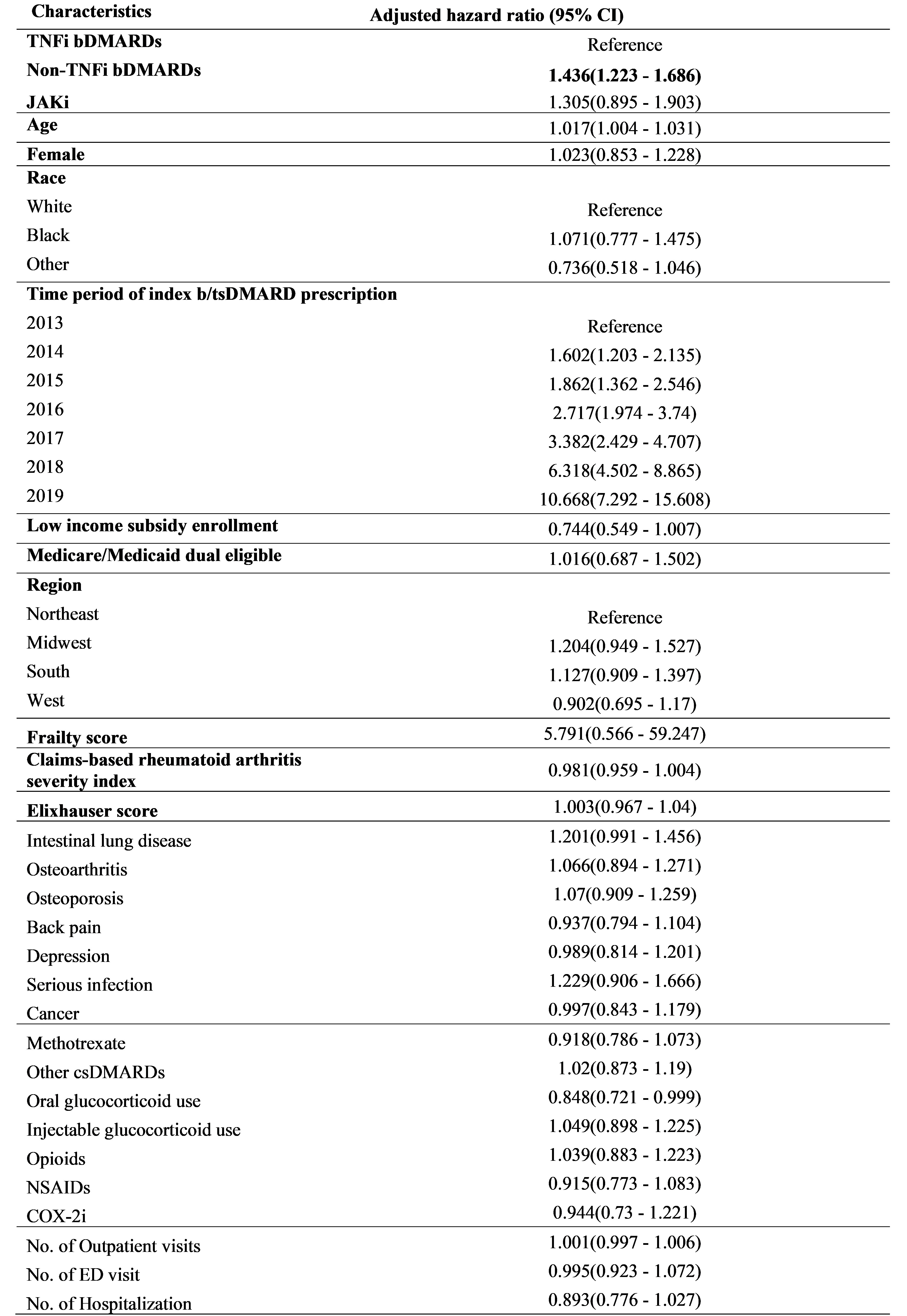
Results: The study cohort included 3,927 patients with RA (mean [SD] age 73 [6]; 2,953 (75%) female). The majority were TNFi initiators (2,263, 57.6%), followed by non-TNFi bDMARDs initiators (1,504, 38.3%), and JAKi initiators (160, 4.1%). Overall, 725 patients (18.5%) discontinued the index btsDMARDs and the average time to discontinuation was 1,110 ±670 days. Multivariable Cox PH model revealed as compared to TNFi bDMARDs, discontinuation was higher for non-TNFi bDMARDs (an adjusted hazard ratio (aHR) 1.4; 95% CI 1.2–1.7), but similar for the JAKi (aHR 1.3; 95% CI 0.9–1.9).
Conclusion: Almost one in five patients discontinued their first treatment with b/tsDMARDs in Medicare beneficiaries with RA. Variation of risk of discontinuation was observed by the different type of b/tsDMARDs, with TNFi bDMARDs the lowest rate of discontinuation. Future research needs to explore the reasons underlying the risk of discontinuation and clinical consequence after discontinuation, with a goal to develop individualized treatment to optimize RA care.
To cite this abstract in AMA style:
Huang Y, Bazzazzadehgan S, Bruera S, Agarwal S. Discontinuation of Targeted Disease-Modifying Antirheumatic Agents in Older Patients with Rheumatoid Arthritis: Retrospective Analysis of Medicare Data [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/discontinuation-of-targeted-disease-modifying-antirheumatic-agents-in-older-patients-with-rheumatoid-arthritis-retrospective-analysis-of-medicare-data/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/discontinuation-of-targeted-disease-modifying-antirheumatic-agents-in-older-patients-with-rheumatoid-arthritis-retrospective-analysis-of-medicare-data/