Session Information
Date: Saturday, November 16, 2024
Title: RA – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Of all approved biologic therapies, other than anti-CD20 depletion only CTLA4-Ig/abatacept treatment reduces levels of pathologic autoantibodies. Herein, our primary goal has been to elucidate the effect of co-stimulatory blockade on the representation of lymphocytes and determine if there are shifts in clonal dynamics associated with clinical response.
Methods: Biologic-naïve patients with seropositive RA were enrolled, and received the standard IV regimen of abatacept, which was then discontinued after the month 5 infusion. Clinical evaluation and biospecimen collection were performed at baseline, 3, 6, and 9 months or when a flare occurred. In addition to routine clinical lab testing, autoantibody responses were evaluated in including CCP3. PBMCs were interrogated at a single cell level for high-dimensional surface phenotype and transcriptomic profiles, by Expanded Cellular Indexing of Transcriptomes and Epitopes (eCITE) sequencing.
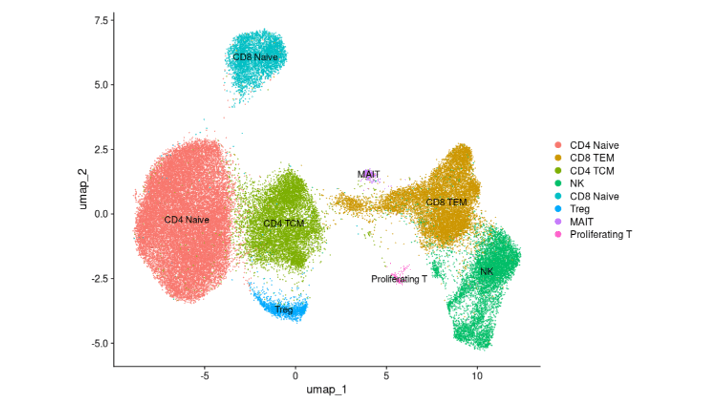
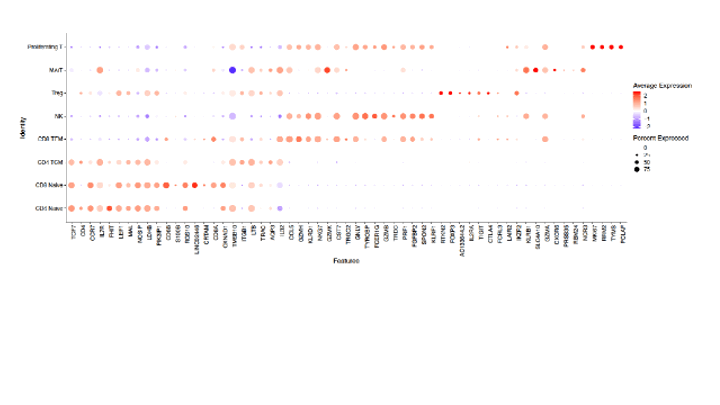
Results: 16/18 RA patients that completed 5 months of abatacept treatment, had complete clinical responses based on CDAI, mirrored by reductions in serum RF and ACPA levels (not shown). Single-cell analyses of 7 patients demonstrated treatment-induced increases in naïve CD4+ T cells, and reductions in regulatory T cells, and CD8+ effector memory cells (Fig.1&2). Treatment was also associated with significant reductions of CD19+sIgD-CD27- (DN2) B cells, CD19+CD69+IgM+IgD+ activated Naïve (aNav) B cells and antibody-secreting cells (ASC) that are linked to pathologic autoimmune B-cell responses (not shown).
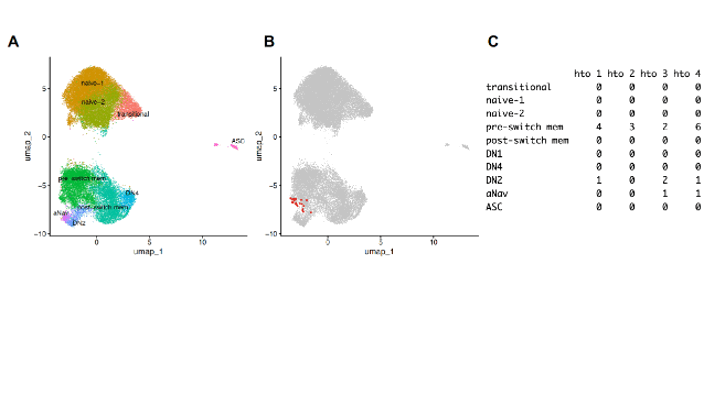
In the subject with the greatest number of single CD19+ B cells, antibody gene usage was examined, which demonstrated a remarkable expansion of an unique B-cell clonotypic set, as identical VH and VL were found in 21 different B cells (Fig 3), distributed across IgM+IgD- pre-switch memory, CD69+IgM+IgD+ activated naïve (aNav) and CD27-CD11c+Tbet+ DN2 detected across all four sampled timepoints. Most were identified as preswitch memory, which was numerically greatest at baseline (Fig3), then decreased after 3treatment, and then expanded at the time of flare (Fig 3C), yet absent in transitional, naïve, post-switch DN1 and DN4 subsets and ASC. VH genes were closest to VH3-33*04 DH6-13*01 JH3*02, with 7 silent and 6 replacement (including a single CDR) mutation. Vk genes was closest to Vk3-15*01 Jk1*01 with 1 silent and 5 replacement (including a single CDR) mutations. As a recombinant IgM, there was strong binding to CCP3 peptide used for clinical diagnosis, citrullinated α-enolase, as well autoreactivity for nucleosome and Sm antigen (not shown), reflecting the broad breach in clonal immune tolerance.
Conclusion: Complete clinical response to abatacept in RA was associated with quantitative reductions in extrafollicular; aNav, DN2, preswitch memory, and ASC, as well as reductions in the B-cell clonotypic expansion in these subsets, as identified by surface phenotype and transcriptomic profiles. Our findings are consistent with abatacept causing preferential therapeutic effects on extrafollicular B cells that include DN2 cells, recently implicated as major RA-associated autoantibody producers in rheumatoid joints.
Supported in part by funds from BMS.
To cite this abstract in AMA style:
Shwetar J, Rigby W, Skopelia-Gardner S, Armarnani A, Ruggles K, Silverman G. Co-stimulatory Blockade Causes Targeted Quantitative and Clonotypic Contractions in Extrafollicular B-cell Subsets in Seropositive RA Patients [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/co-stimulatory-blockade-causes-targeted-quantitative-and-clonotypic-contractions-in-extrafollicular-b-cell-subsets-in-seropositive-ra-patients/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/co-stimulatory-blockade-causes-targeted-quantitative-and-clonotypic-contractions-in-extrafollicular-b-cell-subsets-in-seropositive-ra-patients/