Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Systemic juvenile idiopathic arthritis (SJIA) is a severe disease with symptoms of fevers, rash, and arthritis. High levels of the inflammatory cytokines interlukin-1 (IL-1) and interlukin-6 (IL-6) contribute to the systemic features. In SJIA, IL-1 and IL-6 blocking biologics are lifesaving medications, yet data suggests these medications may have an association with eosinophilia and severe drug reactions. In this study, we sought to clarify the relationships between eosinophilia and IL-1 and IL-6 blocking biologic medications using an electronic health record (EHR) derived SJIA cohort.
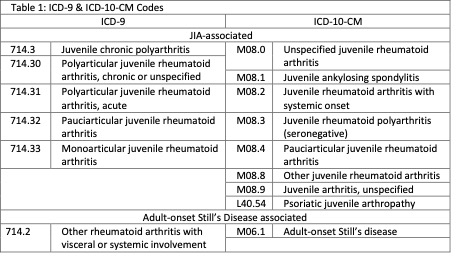
Methods: SJIA patient charts were identified using JIA and adult-onset Still’s disease associated ICD-9 and ICD-10-CM codes (Table 1). Manual review identified charts with inclusion criteria of ≥ 2 rheumatology encounters documenting SJIA and symptom onset ≤ 16 years old. Manual review identified clinical documentation at time of SJIA diagnosis. Demographic, medication, laboratory, and ICD code data were extracted. The first SJIA-associated ICD code approximated the first encounter for SJIA. Eosinophilia was defined as an absolute eosinophil count ≥ 500 cells/microliter. An eosinophilia cluster was defined as eosinophilia within a three-month timespan. IL-1 blocking biologics (anakinra, canakinumab) and IL-6 blocking biologic (tocilizumab) exposure was determined by prescribing data. Statistical analysis was performed by Mann-Whitney and Fisher’s exact testing.
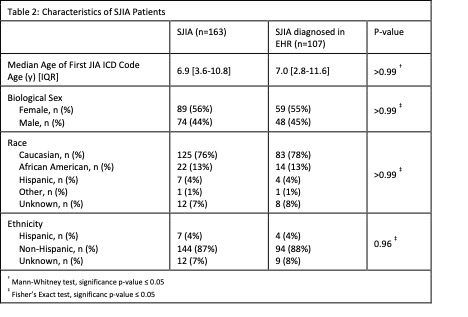
Results: We identified 163 SJIA patients (Table 2). An eosinophilia cluster occurred in 77 patients (47%), with 32 patients (20%) having multiple eosinophilia clusters. Exposure to IL-1 or IL-6 blocking medications occurred in 106 patients (65%) of which 54 (33%) also had eosinophilia.
Clinical documentation was available at the time of SJIA diagnosis for 107 patients (Table 2). In these patients, 54 patients (50%) had an eosinophilia cluster, and of these 29 were within 1 month of SJIA diagnosis. Exposure to IL-1 or IL-6 blocking biologics occurred in 73 patients (68%), of which 54 (74%) occurred within 1 month of SJIA diagnosis. Both biologic exposure and eosinophilia occurred in 40 (37%) patients. Eosinophilia in SJIA patients with and without biologic medication exposure was not significantly different (p 0.22). The first eosinophilia cluster occurred before IL-1 or IL-6 blocking biologic exposure in 15 patients (38%) and during or after medication exposure in 25 patients (62.5%). Eosinophilia clusters occurred within 1 month of the first IL-1 or IL-6 blocking biologic exposure in 25 patients, and of these 23 were within 1 month of SJIA diagnosis.
Conclusion: We identified a SJIA cohort in an EHR with extensive longitudinal clinical data and efficiently extracted data to identify eosinophilia and IL-1 and IL-6 blocking biologic exposure. Eosinophilia and biologic medication exposures were common at the time of SJIA diagnosis. Thus far, significant relationships between SJIA patients with IL-1 or IL-6 blocking biologic use and eosinophilia have not been identified. Studies in SJIA subpopulations are needed to determine if eosinophilia varies by clinical characteristics.
To cite this abstract in AMA style:
Dickey R, Dhar D, Gangireddy S, Ong H, Wei W, Patrick A. Eosinophilia and Exposure to IL-1 and IL-6 Blocking Biologic Medications in a Systemic Juvenile Idiopathic Arthritis Patient Cohort [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/eosinophilia-and-exposure-to-il-1-and-il-6-blocking-biologic-medications-in-a-systemic-juvenile-idiopathic-arthritis-patient-cohort/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/eosinophilia-and-exposure-to-il-1-and-il-6-blocking-biologic-medications-in-a-systemic-juvenile-idiopathic-arthritis-patient-cohort/