Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Enthesitis is a characteristic feature of enthesitis related arthritis (ERA) but can be found in other juvenile idiopathic arthritis (JIA) subtypes. It occurs with greater frequency in juvenile ERA than in adult-onset ankylosing spondylitis. Enthesitis indices exist for adults with spondyloarthritis, but there is no such measure for pediatric patients. This introduces the potential for variability in assessing for enthesitis and diagnosing enthesitis. We aimed to determine the frequency of and indications for assessing enthesitis in clinical practice in addition to the sites most assessed and what factors contribute to the diagnosis of enthesitis.
Methods: A survey was developed and refined by members of the CARRA Juvenile Spondyloarthritis (JSpA) Workgroup. The survey was then further refined using input from experts at the CARRA JIA Forum and through pilot testing. Survey questions included demographics, practice patterns around assessment of enthesitis, and knowledge of enthesitis. The survey was distributed electronically to CARRA members with reminders sent every 2 weeks over a 2-month period. Responses were analyzed using descriptive statistics.
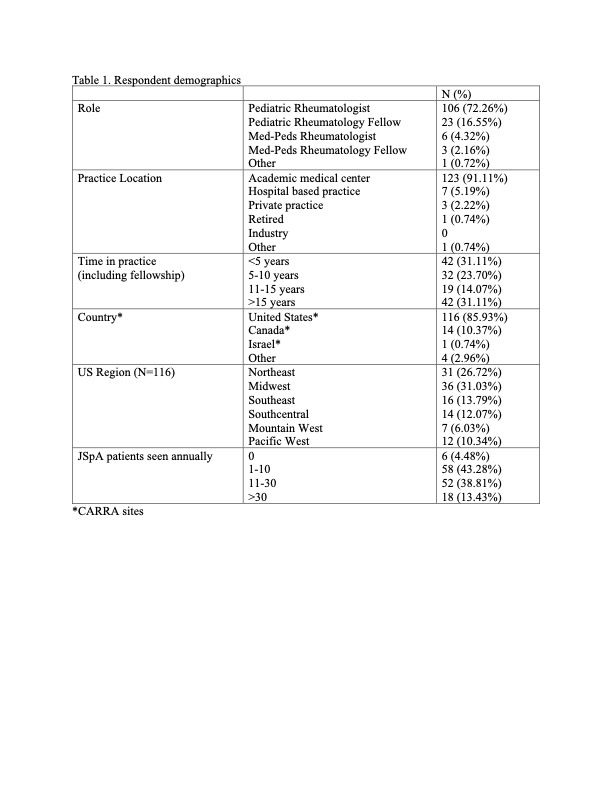
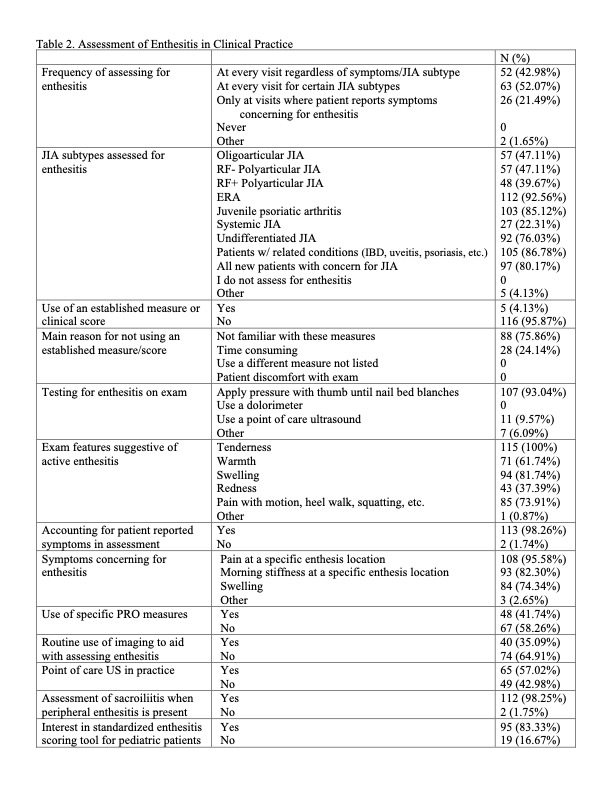
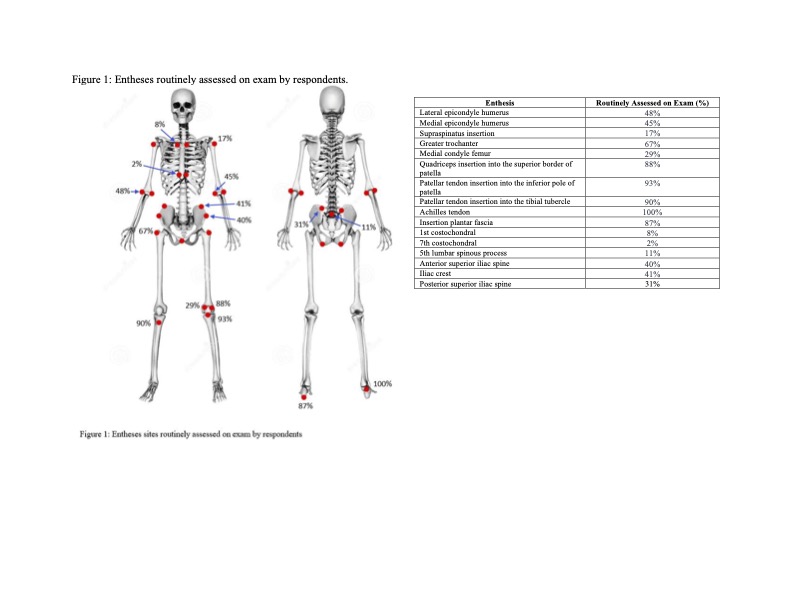
Results: Of the 343 who opened the survey, 139 responded (40.5% response rate). Respondents were predominantly pediatric rheumatologists (76.3%) at academic medical centers (91.1%) in the US (86.0%) (Table 1). Providers were asked in which scenarios they routinely assess enthesitis, and the top answers were patients with ERA (92.6%); patients with HLA B27 associated conditions such as uveitis, psoriasis and inflammatory bowel disease (86.8%); JPsA (85.1%); new patients with concern for JIA (80.1%); and undifferentiated JIA (76.0%). Only 4% of respondents use an established enthesitis scoring measure, but most are interested in a scoring tool being created for pediatric patients (83.3%). The most assessed sites for enthesitis were in the lower extremities with the Achilles tendon being assessed by all respondents (Figure 1). Reported features most concerning for enthesitis were tenderness (100%), swelling (81.7%), and pain with motion/heel walk/squatting (73.9%).
Conclusion: Most providers are interested in a pediatric-specific enthesitis scoring system. There are similarities in current practice patterns in clinical enthesitis evaluation with a higher likelihood of checking for enthesitis in certain JIA subtypes with entheseal sites in the lower extremities most commonly evaluated. Future steps include development of a pediatric enthesitis scoring tool that would standardize enthesitis evaluation and could be used as both a clinical and research tool.
To cite this abstract in AMA style:
Treemarcki E, Tse S, Klein-Gitelman M, Mayer A, Srinivasalu H, Walters H, Oliver M. Assessment of Enthesitis by Pediatric Rheumatology Providers [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/assessment-of-enthesitis-by-pediatric-rheumatology-providers/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/assessment-of-enthesitis-by-pediatric-rheumatology-providers/