Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: The Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry is a large observational registry of children and young adults with childhood-onset rheumatic disease. The Registry is leveraged for various types of research studies including comparative effectiveness work. We assessed the impact of the funded registry sub-study “Start Time Optimization of Biologics in Polyarticular JIA”(STOP-JIA1) on critical element data capture rates and adherence to a declared Consensus Treatment Plan (CTP) as compared to Registry data for patients with polyarticular juvenile idiopathic arthritis (polyJIA) who were not enrolled in STOP-JIA.
Methods: We assessed existing CARRA Registry data for patients with polyJIA enrolled ≤7 days after starting therapy between July 1 2015 – December 1 2022. We separately assessed polyJIA patients enrolled in STOP-JIA (December 1 2015 – August 1 2018). Starting April 1 2020, the Registry began collecting site declaration of polyJIA CTP2 use.
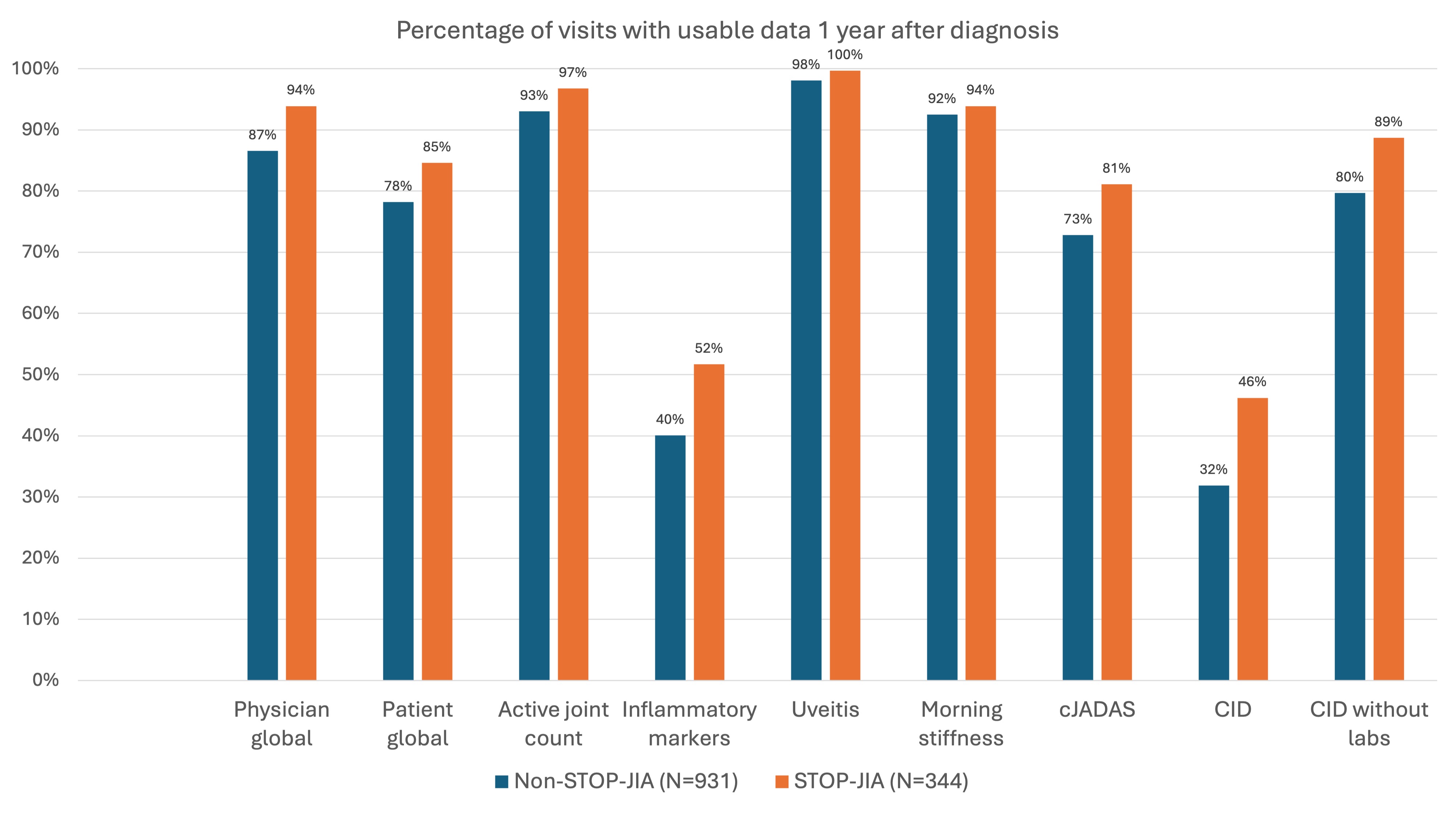
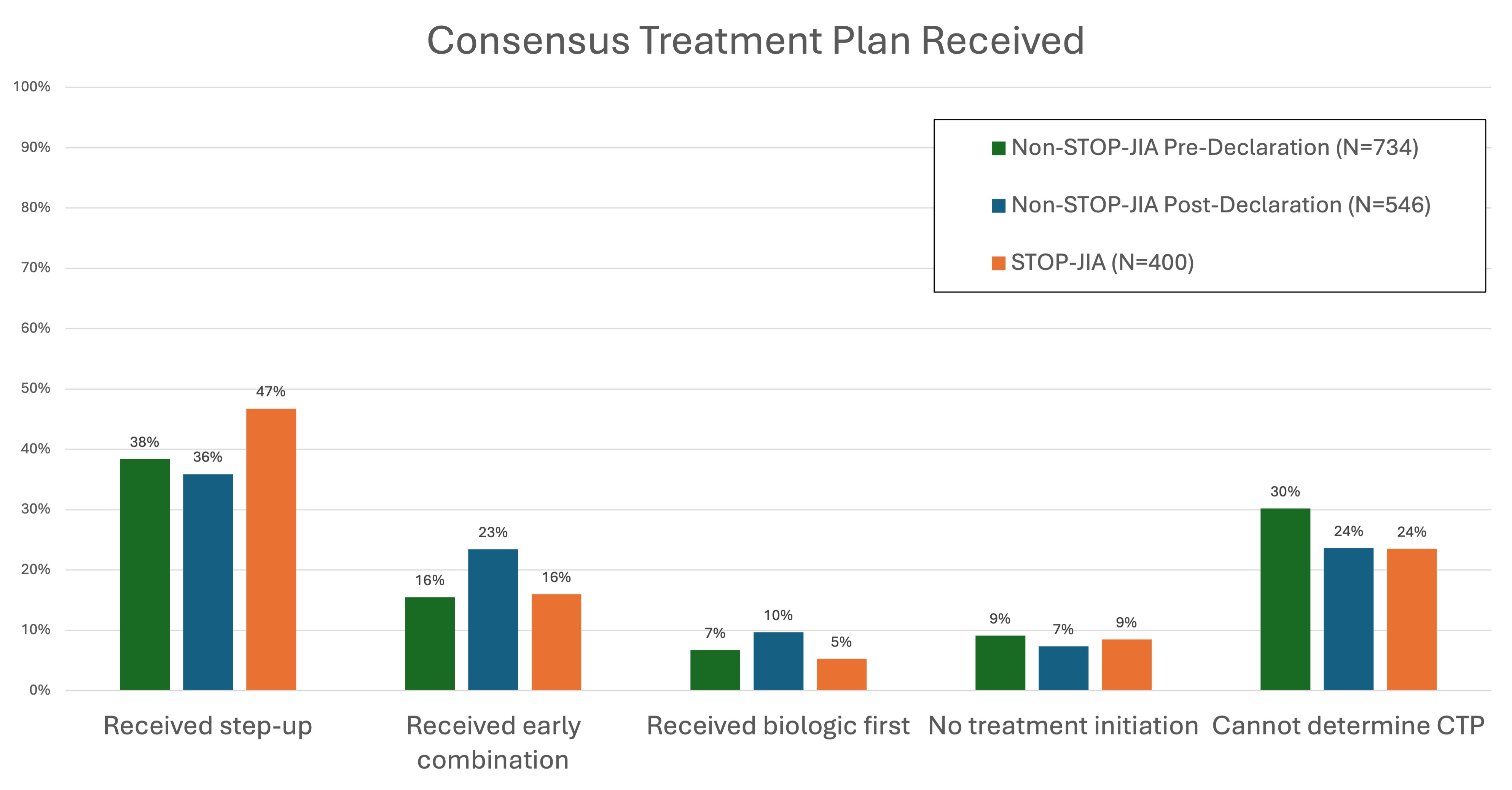
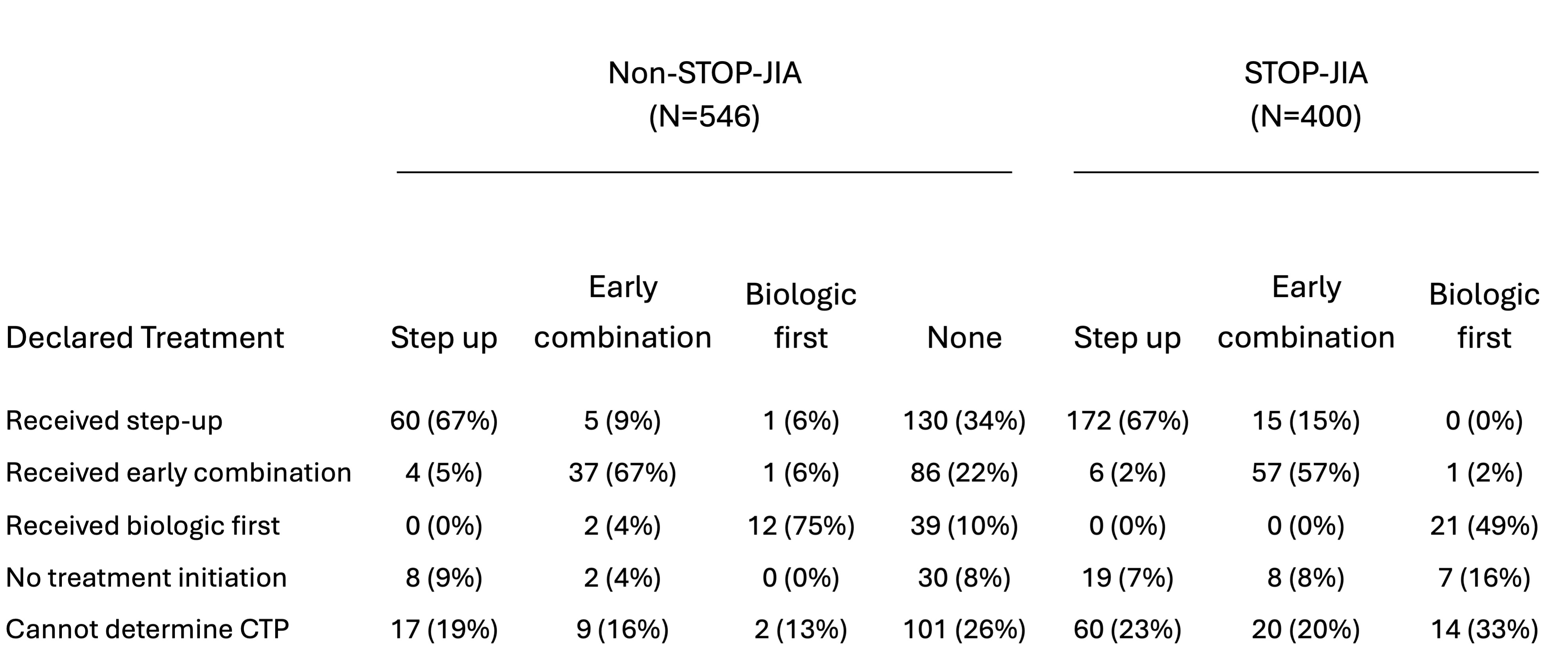
Results: The completeness of outcome data at 1 year after enrollment was generally comparable between STOP-JIA and non-STOP-JIA patient visits across most domains (Figure 1). Percent difference in completeness between groups was 2% – 14%. Reporting of laboratory values was poor across both groups (STOP-JIA 52%, non-STOP-JIA 40%). The 3-variable clinical Juvenile Arthritis Disease Activity Score was calculatable for 81% of STOP and 73% non-STOP patients. Despite introduction of a CTP declaration variable, 70% of Registry patients did not have a declared CTP. Adherence to a declared CTP was not substantially different for patients enrolled in STOP-JIA (Table 1). Across all polyJIA Registry patients, 30%-40% of those with a declared CTP did not appear to receive treatment consistent with the stated CTP. Treatment received was not clearly influenced by presence of the declaration variable or enrollment in STOP-JIA (Figure 2).
Conclusion: This study demonstrates overall high capture rate of critical outcome variables within the CARRA Registry for polyJIA patients. However, completion rates of composite disease activity measure (cJADAS) and lab components were lower than expected. Potential barriers to completion include timeliness of laboratory testing or challenges with data entry. The value and accuracy of the current CTP declaration variable is unclear; patients often do not receive the declared CTP and treatment patterns do not appear to differ substantially between patients with and without a CTP declaration. The reasons for this difference are likely multifactorial and deserve further exploration. Analysis of the STOP-JIA Registry subset study shows that enhanced funding for data completeness may need to be optimized to guarantee higher variable capture rates. Reassuringly, treatment patterns in non-STOP-JIA patients were like those enrolled in STOP-JIA, suggesting that Registry subset studies are reflective of real-world treatment patterns. Future investigations of root causes of sub-optimal data capture are critical to ensuring ongoing value of Registry data collection.
1. Kimura Y et al. Arthritis Rheumatol. 2021 Oct;73(10):1898-1909.
2. Ringold S et al. Arthritis Care Res (Hoboken). 2014 Jul;66(7):1063-72.
To cite this abstract in AMA style:
Glaser D, Yildirim-Toruner C, Tarvin S, Ardoin S, Beukelman T. Variability of Data Completeness and Consensus Treatment Plan Adherence for Polyarticular Juvenile Idiopathic Arthritis Patients Within the Childhood Arthritis and Rheumatology Research Alliance Registry [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/variability-of-data-completeness-and-consensus-treatment-plan-adherence-for-polyarticular-juvenile-idiopathic-arthritis-patients-within-the-childhood-arthritis-and-rheumatology-research-alliance-regis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/variability-of-data-completeness-and-consensus-treatment-plan-adherence-for-polyarticular-juvenile-idiopathic-arthritis-patients-within-the-childhood-arthritis-and-rheumatology-research-alliance-regis/