Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
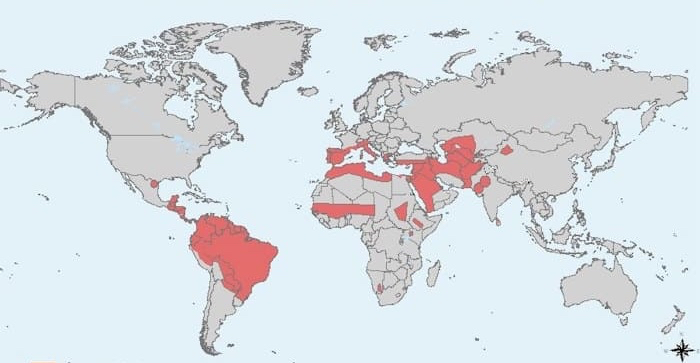
Background/Purpose: Leishmaniasis is an infectious disease caused by protozoon that occurs endemically in the Mediterranean Basin and South America and can affects to travellers to this areas (Figure 1). Leishmaniasis disease can manifest as cutaneous (CL), mucocutaneous (ML) or visceral (VL) disease (1). The number of infections produced by leishmaniasis in patients being treated with anti-TNF inhibitor drugs has increased in the last recent years (1,2)
The aim of the present study was to evaluate the risk of leishmania infection as well as its evolution in immunosuppressed patients with anti-TNF therapy in leishmania endemic areas.
Methods: A descriptive, retrospective, multi-center study was performed in two hospital in the southeast of Spain. Data was collected from a microbiology database from patients with positive biopsy for leishmaniasis from january 2020 to december 2023. Variables analyzed were: age, gender, presence of immunosuppressive disease and presence of biological treatment (BT) at the time of diagnosis of leishmaniasis. The treatment received for leishmaniasis and the disease outcome were also reviewed.
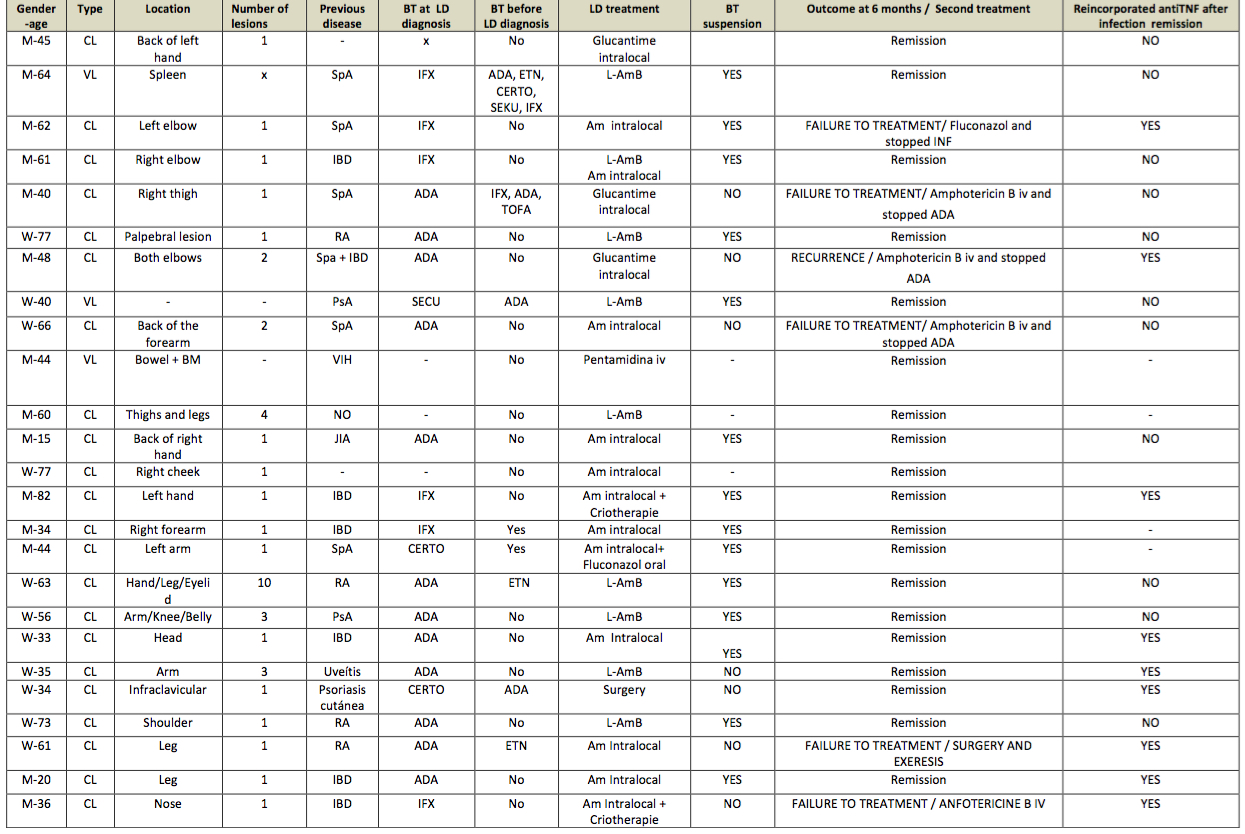
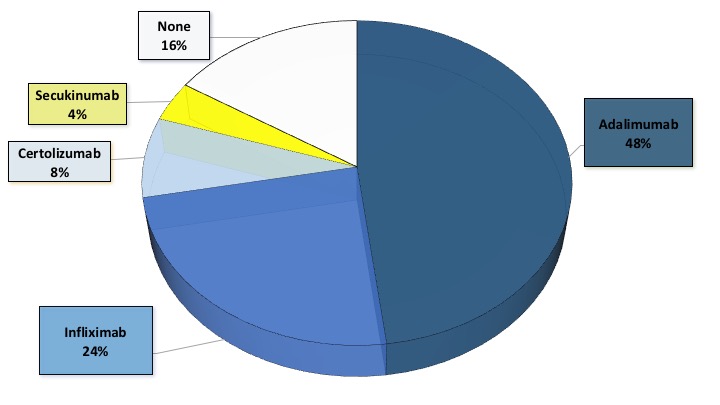
Results: A total of 25 patients with leishmaniasis were included, whose characteristics are summarized in a table (Table 1). Of the 25 patients, 20 were on antiTNF therapy and one of them was receiving antiL17 treatment (Figure 1), however this patient had received antiTNF recently. Most patients with antiTNF treatment had CL (92%) and 2 patients had VL (2%). Immunosuppressive treatment was stopped in all patients when they were diagnosed with leismaniasis, except for 7 patients with CL. Five patients who did not discontinue antiTNF had primary failure to local treatment and recurrence of infection. These five patients subsequently stopped antiTNF and four of them required amphotericin B intravenous until remission infection and another one received surgery with remission. Of these seven patients with primary faillure to leishmaniasis treatment, four reincorporated antiTNF treatment.
In the present study, in total nine patients reintroduced treatment with antiTNF therapy when they were in remission of leishmaniasis and continue in remission 12 months after reintroducing antiTNF therapy.
Conclusion: AntiTNF therapy is a potential risk for leishmaniasis disease in endemic areas. Our treatment recommendations included discontinuations of anti TNF until recovery and systemic treatment. The risk of restarting anti-TNF after recovery is unclear.
References:
1. Bosch-Nicolau P et al.Leishmaniasis and tumor necrosis factor alpha antagonists in the Mediterranean basin. A switch in clinical expression. PLoS Negl Trop Dis. 2019
2. Hammarström H et al. Leishmania infantum infection after visiting southern Spain in patients on biological treatment; an observational, longitudinal, cohort study. Travel Med Infect Dis. 2023
To cite this abstract in AMA style:
Albaladejo Paredes G, Pérez González A, Monleón Acosta E, Rodríguez Fernández J, Manuel Hernández P, Fernández Díaz C, Oliva Ruíz M, Andreu Ubero J, Castillo Dayer P, Soriano Navarro E, Pérez Parra D, Capozzi C, González Molina M, Orts Paco J, Serrano Serra J, Cogolludo Campillo V. Anti-TNF Therapy as a Potential Risk of Leishmania Infections [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/anti-tnf-therapy-as-a-potential-risk-of-leishmania-infections/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/anti-tnf-therapy-as-a-potential-risk-of-leishmania-infections/