Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Patients with autoimmune rheumatic diseases (ARDs) are at high risk of herpes zoster (HZ) due to their condition and treatment. The new recombinant vaccine against HZ (RZV, Shingrix®) offers safety improvements. However, existing literature includes only two prospective studies with small samples, limiting definitive conclusions about the influence on underlying disease activity and the impact of drugs on vaccine response. This study aims to evaluate disease safety of RZV in ARD patients compared to non-vaccinated ARDs (primary endpoint) and assess overall safety and humoral immunogenicity compared to a non-immunosuppressed control group (CG) (secondary endpoints).
Methods: This is a prospective randomized placebo-controlled phase 4 study of ARD patients at high risk of HZ. ARD patients ( >18 years) were randomized into two groups: P1 (vaccine) and P2 (placebo). Non-immunosuppressed subjects served as CG. CG and P1 received two intramuscular doses of RZV 6 weeks apart on D0 (V1) and D42 (V2), while P2 received placebo. Disease activity was evaluated by activity scores. Blood samples were collected before the 1st dose (V1) and 6 weeks after the 2nd dose (V3). Adverse events were assessed by a standardized questionnaire. Humoral immunogenicity was measured via anti-gE antibody serum concentrations using an in-house ELISA. Humoral response to RZV was defined as anti-gE antibody concentration ≥4-fold the lower limit of detection (0.02 mIU/mL) in initially seronegative subjects and as an antibody concentration ≥4-fold the pre vaccination concentration in initially seropositive subjects ( >0.02 mIU/mL). GMT was calculated from ln-transformed concentrations.
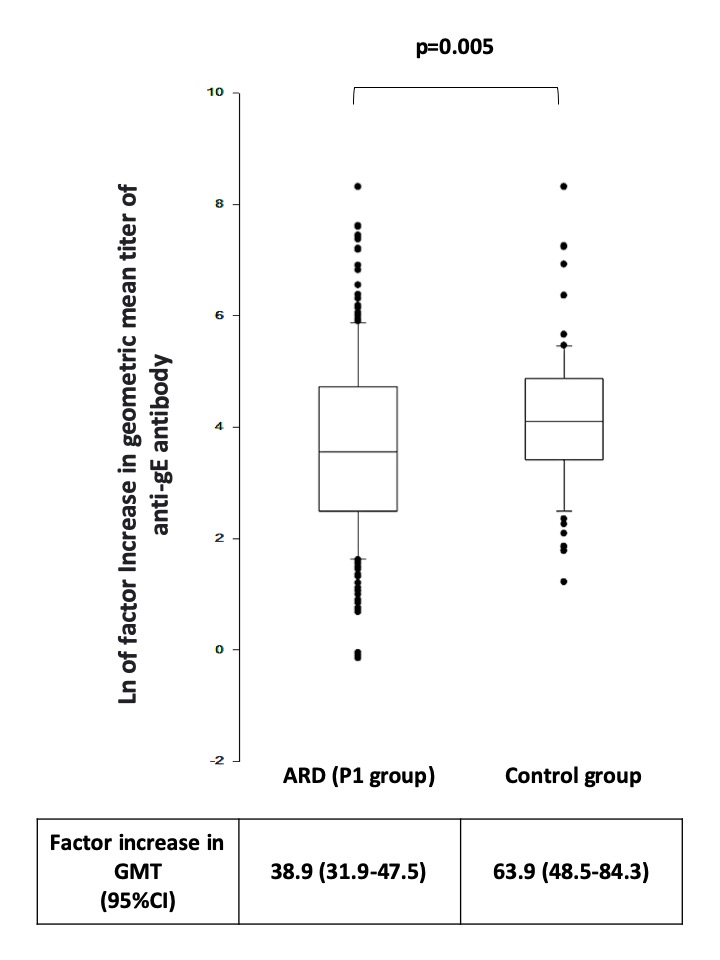
Results: Of the 1,180 ARD patients and 393 CG to be achieved by the end of study, 662 patients (P1: 324 and P2: 338) and 86 CG had full data for the first 12 weeks (V1-V3) and were included in this interim analysis. ARDs included 9 diseases, primarily rheumatoid arthritis (27%) and systemic lupus erythematosus (25%). Treatments were prednisone (37%), immunosuppressive (79%), and biologic therapy (45%). P1 (vaccine) and P2 (placebo) groups were balanced for mean age (p=0.785), ARD diagnoses (p >0.05), and therapies (p >0.05), except for lower mycophenolate mofetil (MMF) in P1 (p=0.038). The primary endpoint showed comparable flare frequencies in P1 and P2 at V2 (13.6% vs. 12.4%, p=0.659) and V3 (11% vs. 12.8%, p=0.485). Secondary endpoints: Safety – no moderate/severe AEs, but higher frequency of AEs in ARD patients (p=0.009); Humoral response in V3 – lower in ARDs than CG (93% vs. 99%, p=0.053). Baseline GMT of anti-gE was similar (p=0.912), but GMT increase after 2 doses was lower in ARD patients than CG (38.9 [95% CI 31.9-47.5] vs. 63.9 [95% CI 48.5-84.3]; p=0.005). Multivariate analysis revealed that rituximab (RTX) (p=0.003) and MMF (p=0.017) were the major deleterious factors for reduced vaccine response. No HZ case by RT-PCR up to week 12.
Conclusion: Our data demonstrate that RZV should be recommended for high HZ risk ARD patients due to its good disease safety and adequate short-term immunogenicity. Special surveillance is needed for patients under RTX and MMF, who may require booster dose.(ClinicalTrials.gov NCT05879419)
To cite this abstract in AMA style:
Aikawa N, Medeiros-Ribeiro A, Pasoto S, Kupa L, Seguro L, ASSAD A, Borba E, Saad C, Figueiredo Neves Yuki E, Andrade D, Shimabuco A, Bonfiglioli K, Domiciano D, Moraes J, Shinjo S, Sampaio-Barros P, Giardini H, de Oliveira J, Antonelli I, Pinheiro R, Bonini T, Lopes M, Silva C, Bonfa E. Recombinant Herpes Zoster Vaccine in a Large Cohort of Autoimmune Rheumatic Diseases Patients: An Interim Analysis of a Prospective Randomized Phase 4 Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/recombinant-herpes-zoster-vaccine-in-a-large-cohort-of-autoimmune-rheumatic-diseases-patients-an-interim-analysis-of-a-prospective-randomized-phase-4-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/recombinant-herpes-zoster-vaccine-in-a-large-cohort-of-autoimmune-rheumatic-diseases-patients-an-interim-analysis-of-a-prospective-randomized-phase-4-study/