Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Supplement use among individuals with SLE is not well-characterized, despite potential effects of some supplements on SLE activity or on treatments for SLE or its associated comorbidities. Further, some supplements may interact with important lab values [e.g., vitamin B7 (biotin) interferes with assays for troponin, hormones (e.g., thyroid-stimulating hormone), and vitamin D]. We estimated the prevalence of overall and individual supplement use in a primarily Black U.S. cohort of individuals with SLE.
Methods: Participants were recruited from an ongoing population-based SLE cohort [Georgians Organized Against Lupus (GOAL)] and asked to report their current supplements during a single study visit (10/2019-5/2022). Descriptive statistics were calculated overall and by age, sex, and race, with statistical comparisons by chi-square test. The most common supplements were defined as >5% of participants reporting use. Supplements with potential effects on SLE or treatments or potential interference with lab tests were identified via NIH and FDA sources.
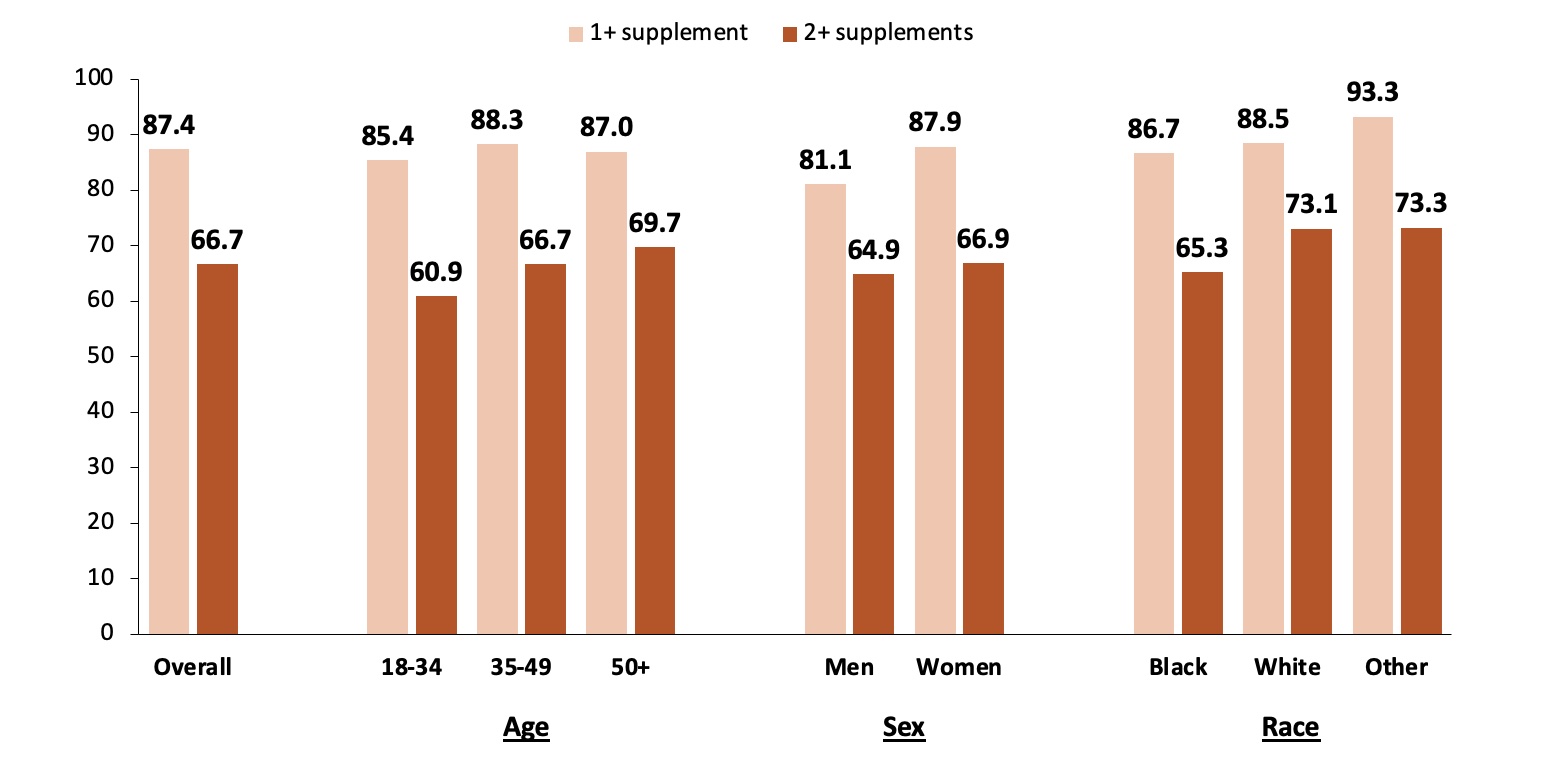
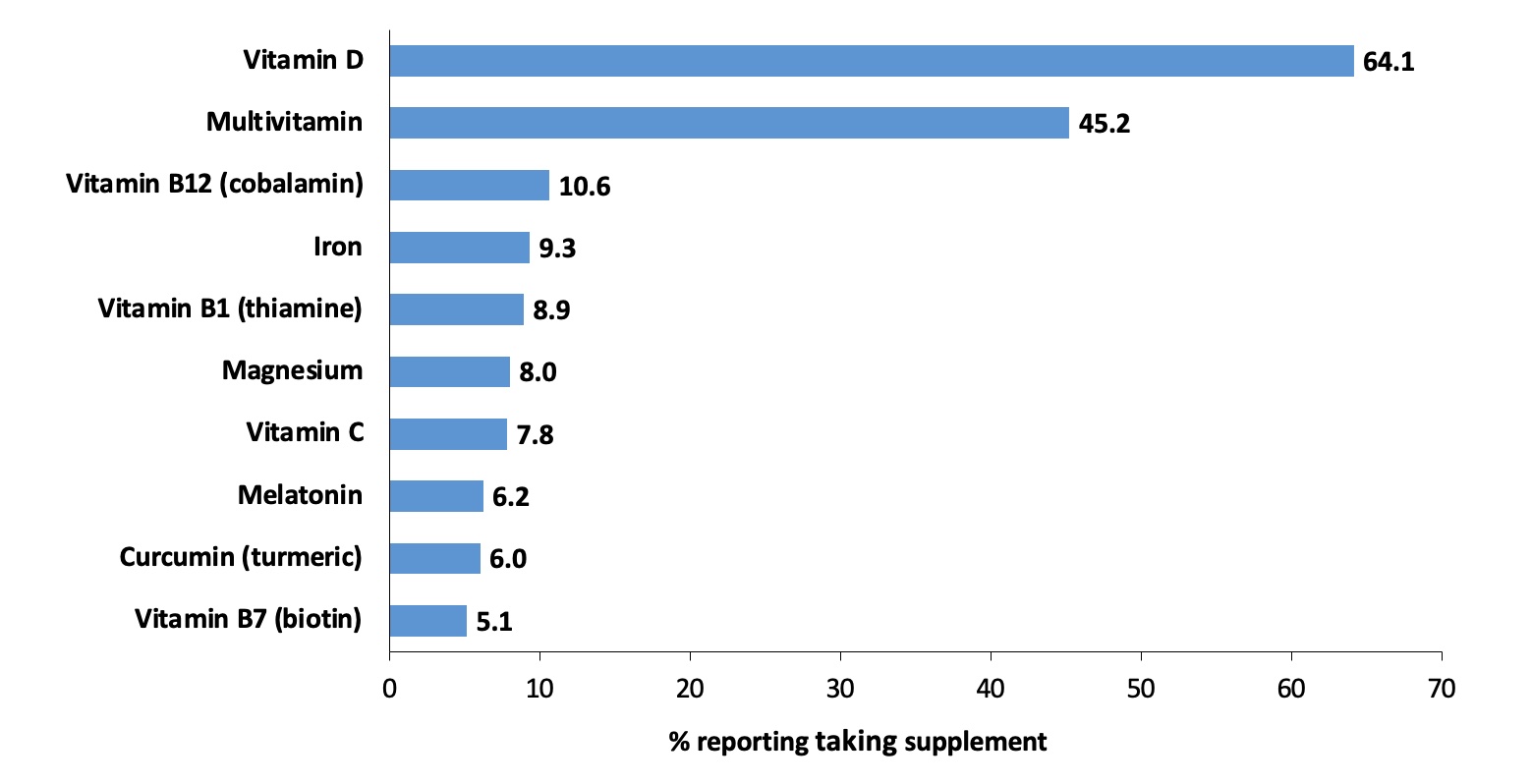
Results: Overall, 87.4% of participants (N=451; mean age, 46.2 years; 91.8% female and 82.5% Black) reported taking at least one supplement. The median number of supplements reported was 2 (25th-75th percentile, 1-3; range, 0-12), and 66.7% of participants were taking two or more supplements. Prevalence estimates did not differ by age, sex, or race (Figure 1). The most commonly reported supplements were vitamin D (64.1%), multivitamins (45.2%), and vitamin B12 (10.6%), as well as other B vitamins (vitamin B1 and biotin), vitamin C, iron, magnesium, melatonin, and curcumin (turmeric) (Figure 2). While less common, some participants were using supplements that have theoretical immune-activating effects, such as ashwagandha (0.4%) and echinacea (0.2%), or have unknown health implications, such as colloidal silver (0.2%).
Conclusion: Supplement use in our cohort was common, with most participants reporting taking at least one supplement and about two-thirds reporting taking two or more. Some supplements (e.g., vitamin D and multivitamin) may be recommended or prescribed by a physician and beneficial in the setting of SLE. However, other reported supplements may pose risks for immune activation (e.g., echinacea) or interfere with lab tests (e.g., biotin). Given the variety of supplements available, the lack of consistent regulation of ingredients, and wide distribution of misinformation, it is very important for physicians to assess supplement use in their patients, encourage patients to use trusted information sources, and be aware of the potential benefits and risks associated with commonly used supplements.
To cite this abstract in AMA style:
Ellyson R, Yazdany J, Lim S, Pearce B, Plantinga L. Supplement Use in a Diverse Cohort of Individuals with SLE [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/supplement-use-in-a-diverse-cohort-of-individuals-with-sle/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/supplement-use-in-a-diverse-cohort-of-individuals-with-sle/