Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Transient receptor potential canonical 1 (TRPC1), a non-selective cation channel, is characterized by its high permeability to calcium ions (Ca2+), a vital second messenger for numerous cellular functions. MSCs can differentiate into multiple cell lineages, including osteoblasts, chondrocytes, and adipocytes. Our prior study showed that mice with global deletion of TRPC1 had higher bone mass, but this was in the setting of the mice having hyperparathyroidism. To further elucidate the role of TRPC1 in modulating bone remodeling homeostasis, we generated MSC-specific TRPC1-deficient mice (TRPC1-KOMSC).
Methods: TRPC1ex-2 flox/flox (f/f) females were crossed with Prrx1Cre+; TRPC1f/f males to generate TRPC1-KOMSC and Cre- negative control mice. At 15 weeks, microCT was performed on male and female tibial trabecular and cortical bones, and dynamic and static histomorphology. Osteoclast numbers (N.Oc.) were quantified by TRAP staining. MSC was quantified by flow cytometry (Ter119–, CD45–, PDGFRα+, Sca1+ and LepR+) and colony forming units (CFU). In vitro, MSCs were differentiated into osteoblasts for 21 days or adipocytes for 7 days using osteogenic and adipogenic specific media, respectively. Differentiation was confirmed with Alizarin Red S and Oil Red O staining for osteoblast and adipocyte.
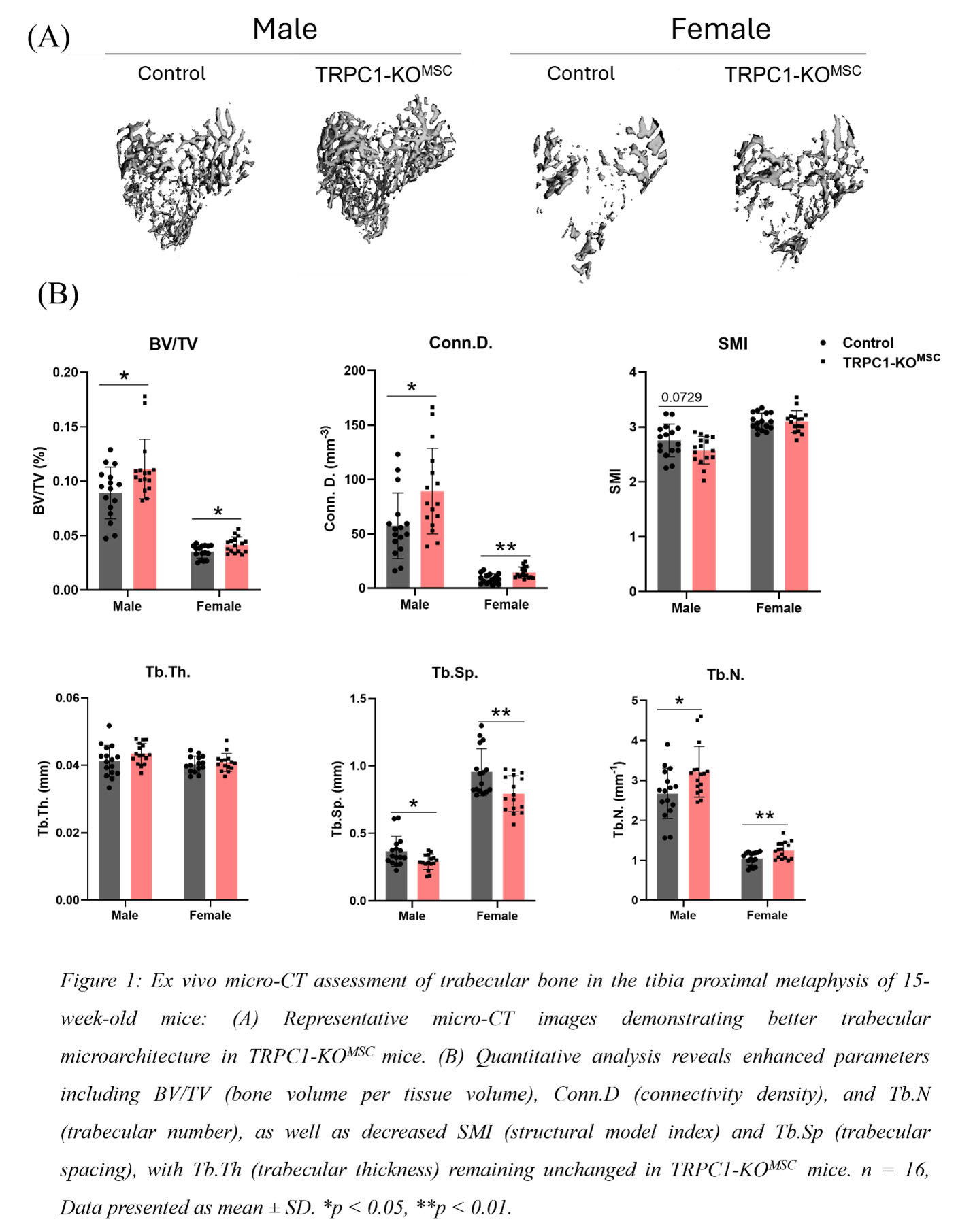
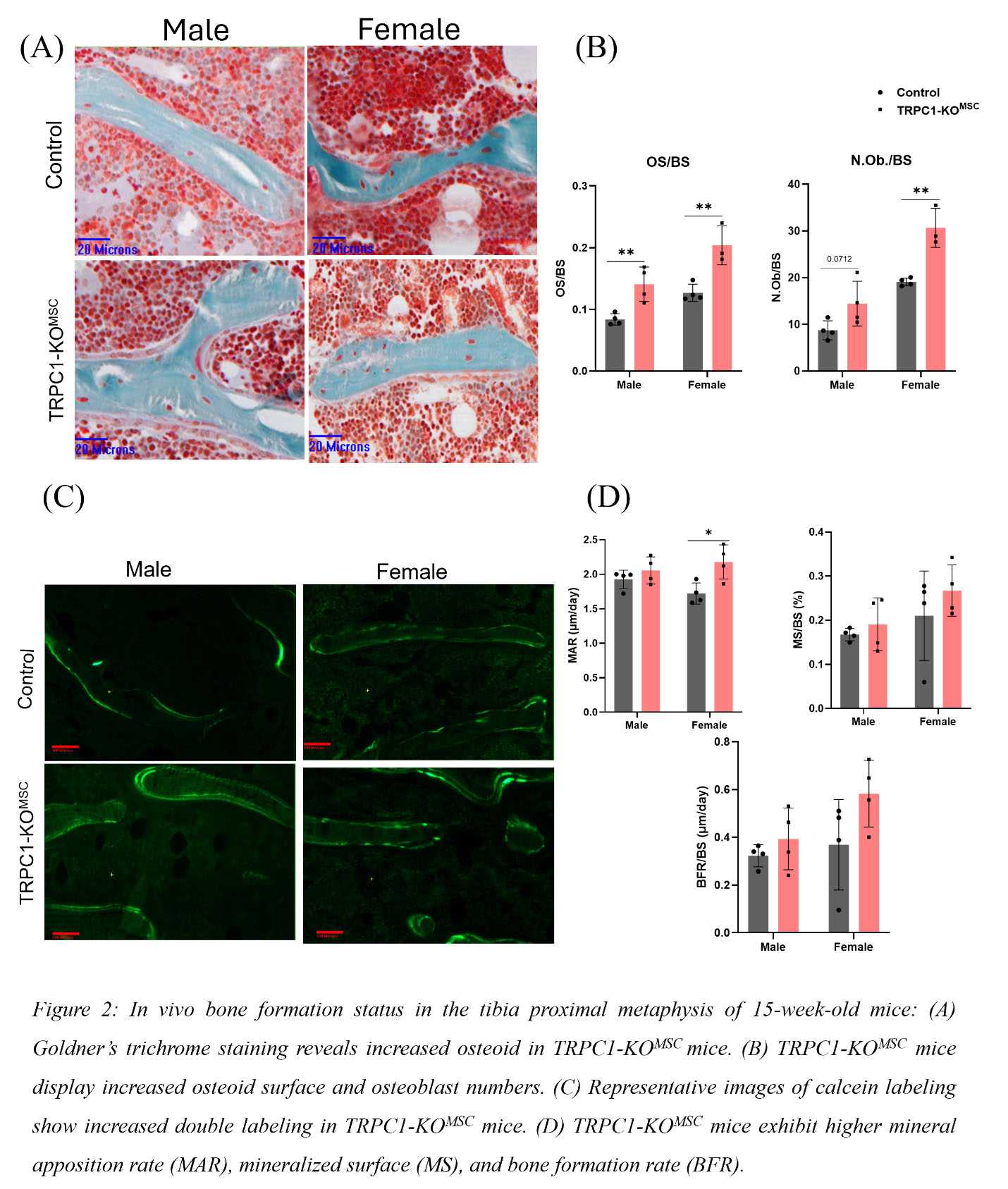
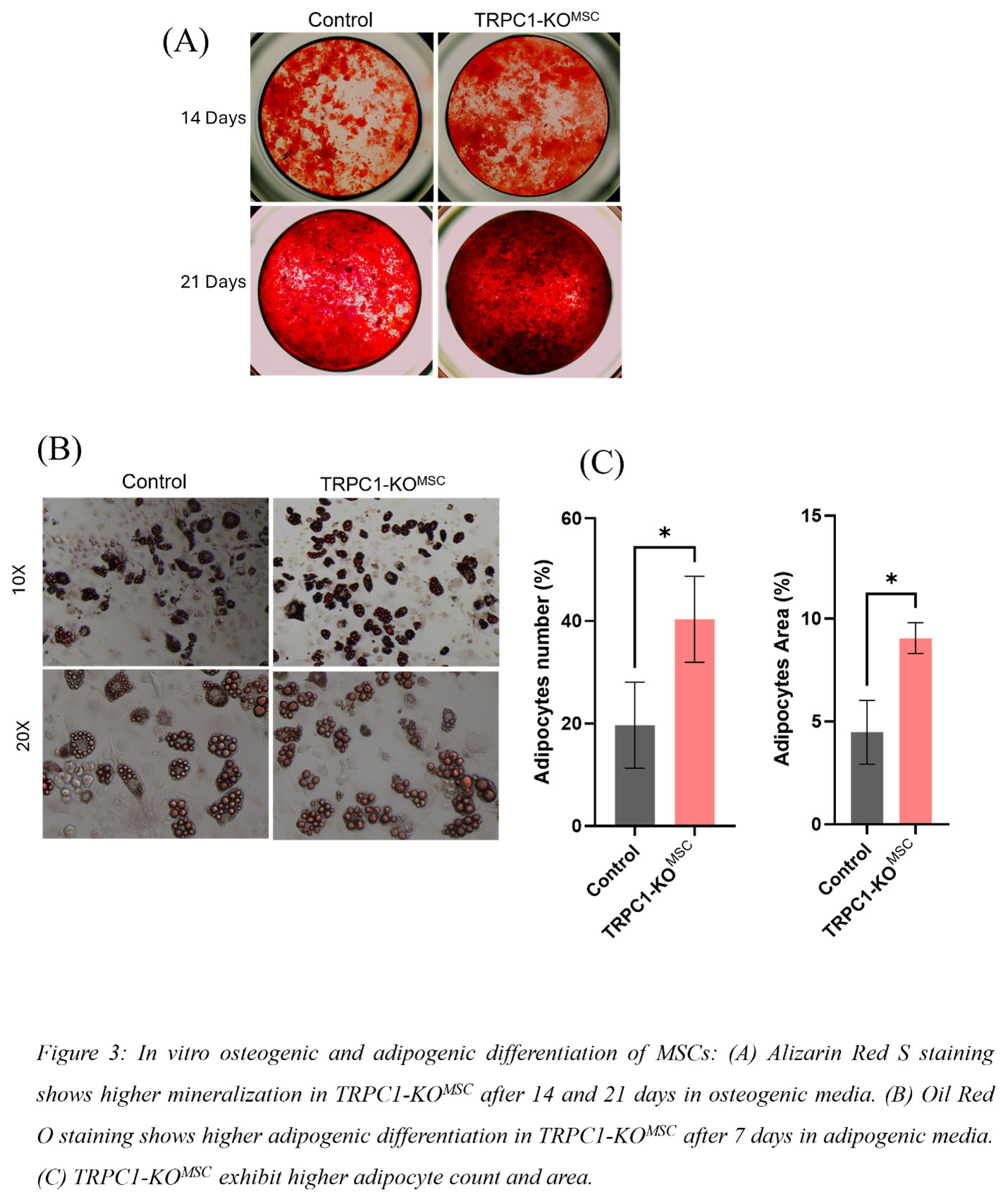
Results: Micro-CT assessment of tibial trabecular bone from 15-week-old male and female TRPC1-KOMSC and Cre negative littermates revealed significantly increased bone volume/tissue volume (BV/TV), connectivity density (Conn.D.), and trabecular number (Tb.N.), and decreased trabecular spacing (Tb.Sp.) (Fig1). Osteoid surface (OS) and number of osteoblasts (N.Ob.) were elevated in TRPC1-KOMSC mice compared to controls (Fig2A). Dynamic bone analysis showed a significant increase in mineral apposition rate (MAR) in female TRPC1-KOMSC mice (Fig2B). No significant differences were observed in cortical bone parameters. Numbers of PDFGRa+/SCa1+ MSC were similar independent of TRPC1 expression, but LepR+ MSC were increased in TRPC1-KOMSC compared to WT mice. The proliferation of MSC assessed by Ki67 staining was significantly decreased in TRPC1-KOMSC compared to WT mice, but CFU was similar. MSC derived from TRPC1-KOMSC mice induced significantly faster OB differentiation than WT MSC. TRPC1-deficient MSC differentiated with adipogenic media formed significantly more mature adipocytes than WT MSC (Fig3A &B).
Conclusion: These studies reveal negative regulation of bone homeostasis by TRPC1 expression in MSC. In vivo, MSC-specific TRPC1-deficiency increases osteoblast numbers, increases osteoid production, and leads to higher bone mass. The osteoclast compartment was unchanged. MSC-specific TRPC1-deficiency leads to increased LepR+/Sca1/PDGFRa+ MSC that have increased commitment to osteoblasts and adipocytes, suggesting that TRPC1 negatively regulates the early MSC differentiation of LepR+ MSC. These findings highlight TRPC1 as a potential therapeutic target for enhancing bone health and treating bone loss-related conditions and bone regeneration.
To cite this abstract in AMA style:
Yadav J, Yadav S, Houser J, Onopuik M, Tsiokas L, Humphrey M. Cation Channel TRPC1 Regulates Mesenchymal Stem Cell Differentiation and Bone Formation [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/cation-channel-trpc1-regulates-mesenchymal-stem-cell-differentiation-and-bone-formation/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cation-channel-trpc1-regulates-mesenchymal-stem-cell-differentiation-and-bone-formation/