Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Previous clinical trials of mycophenolate mofetil (MMF) in SLE were mainly focused on lupus nephritis (LN) (patients have been diagnosed with LN). In the pathogenesis of LN as well as other organ damages, anti-dsDNA antibody plays an important role.
To assess the efficacy and safety of MMF plus hydroxychloroquine (HCQ) and prednisone compared with HCQ and prednisone alone in newly diagnosed and treatment-naïve SLE patients with high titer of anti-dsDNA antibody and without major organ involvement.
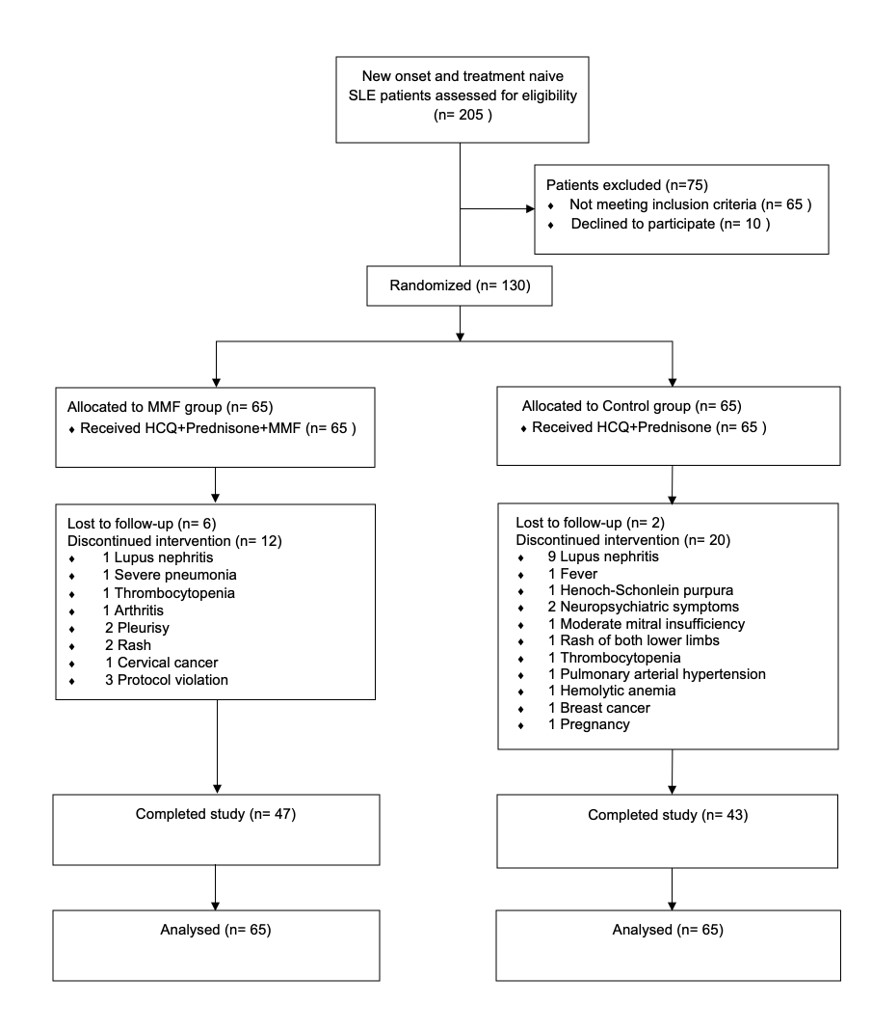
Methods: This investigator-initiated multicenter, open-label, randomized, controlled trial which enrolled 130 patients was conducted in three hospitals across China between September 2018 and September 2021. Inclusion criteria were newly diagnosed and treatment-naïve SLE patients with high titer of anti-dsDNA antibody (anti-dsDNA (ELISA) ≥ 300 IU/mL; (CLFT) ≥1:10) and without major organ involvement (brain, heart, liver, kidney, lung, muscle, and gastrointestinal tract).
Participants were randomly assigned to received HCQ (5mg/kg/day) and prednisone (0.5mg/kg/day) (n=65) or HCQ (5mg/kg/day) and prednisone (0.5mg/kg/day) plus MMF 500mg twice daily (n=65).
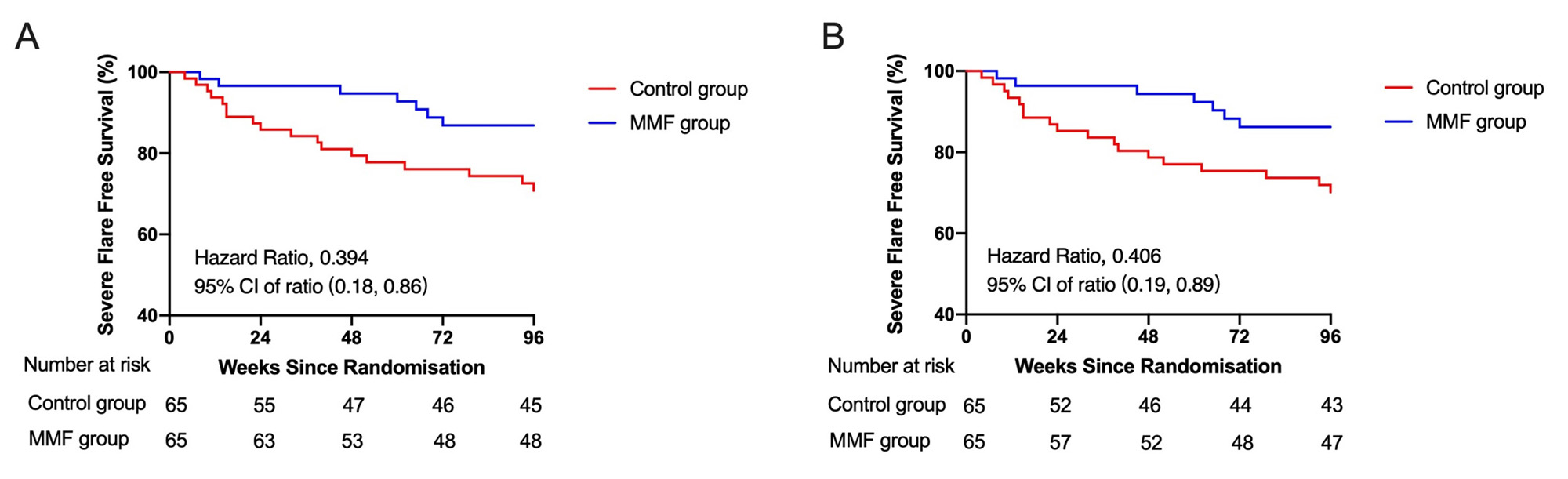
The primary endpoint was the proportion of SLE patients having flares according to SELENA-SLEDAI Flare Index. The secondary outcome included the proportion of lupus low disease activity state (LLDAS) at week 96, short form-36 (SF-36) score before and after treatment, the proportion of adverse events, changes in SLEDAI-2000 score and prednisone dose.
Results: During the follow-up, 10.76% of patients in MMF group and 27.69% of patients in Control group showed severe flares (RR: 0.39, 95% CI: 0.17-0.87, p=0.014). 1.54% of patients in MMF group and 13.85% of patients in Control group manifested LN (RR: 0.11, 95% CI: 0.01-0.85, p=0.008). There was no significant differences in mild to moderate flares between the two groups (p=0.856). Besides, 33.85% of patients in MMF group and 16.92% of patients in Control group never had a relapse in the follow-ups (p=0.027). For the secondary outcomes, 41.54% patients in MMF group and 35.38% patients in Control group achieved LLDAS. SF-36 score of MMF group was better than Control group. Adverse events were reported in 49.23% patients in MMF group and 36.92% patients in Control group (p=0.157). Prednisone dosage was decreased gradually from baseline (mean [SD]: 33.66 [11.89] mg in Control group; 37.93 [13.56] mg in MMF group) to Week 96 (6.30 [6.82] in Control group; 4.88 [3.65] mg in MMF group) (prednisone dose change, p=0.087).
Conclusion: Low dose of MMF might decrease the rate of severe flare and lower the incidence of LN in new-onset SLE patients with high titer of anti-dsDNA antibody and without major organ involvement. Further research is needed to confirm efficacy of longer term outcomes in more diverse patient populations.
To cite this abstract in AMA style:
Ye J, You Y, Zhou Z, Wu J, wang F, Yang C. Safety and Efficacy of Mycophenolate Mofetil in New-onset Systemic Lupus Erythematosus with High Titer of Anti-dsDNA Antibody and Without Major Organ Involvement: A Multicenter Randomized Controlled Trial [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/safety-and-efficacy-of-mycophenolate-mofetil-in-new-onset-systemic-lupus-erythematosus-with-high-titer-of-anti-dsdna-antibody-and-without-major-organ-involvement-a-multicenter-randomized-controlled-t/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-and-efficacy-of-mycophenolate-mofetil-in-new-onset-systemic-lupus-erythematosus-with-high-titer-of-anti-dsdna-antibody-and-without-major-organ-involvement-a-multicenter-randomized-controlled-t/