Session Information
Date: Tuesday, November 14, 2023
Title: (2227–2256) Spondyloarthritis Including Psoriatic Arthritis – Treatment: SpA Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Patients (pts) with psoriasis (PsO) or PsA are at increased risk for developing gout; and hyperuricemia (HU) prevalence is higher in PsO/PsA pts than the general population1,2. A previous analysis showed that the profile of pts with vs without HU was distinct and that the efficacy of secukinumab (SEC) was comparable in hyper- and normo-uricemic pts except for PsO Area Severity Index response (PASI90), which was lower with SEC 150mg in HU pts3. These post hoc analyses sought to contrast PsA pts with vs without HU and evaluate the efficacy of guselkumab (GUS) through 1y in both cohorts.
Methods: Adults in DISCOVER 1&2 (90% bionaive) with active PsA despite standard therapies were randomized 1:1:1 to GUS 100 mg every 4 weeks (Q4W); GUS 100 mg at W0, W4, Q8W; or placebo. HU was defined as baseline (BL) serum urate levels of ≥360 μmol/L. Pts without HU (no HU) but with history of gout or uric-lowering therapies were excluded. Among GUS-randomized pts, the effect of HU vs no HU on changes from BL through W52 in Disease Activity (DA) Index for PsA (DAPSA), PsA DA Score (PASDAS), PASI, Health Assessment Questionnaire Disability Index (HAQ-DI), Short Form 36-item physical component summary (SF-36 PCS) score, and BASDAI (in pts with axial involvement) was assessed with mixed models for repeated measures adjusting for BL outcome levels, GUS regimen, prior TNFi use, and BL conventional synthetic DMARD use. The effect of HU vs no HU on achievement of ACR50, DAPSA low disease activity (LDA; ≤14), PASDAS LDA (≤3.2), PASI90, Functional Assessment of Chronic Illness Therapy – Fatigue (FACIT-F) response (Δ≥4), and enthesitis/dactylitis resolution in pts not in response/with condition at BL (all non-responder imputation for missing data) was assessed with logistic regression adjusting for the same covariates.
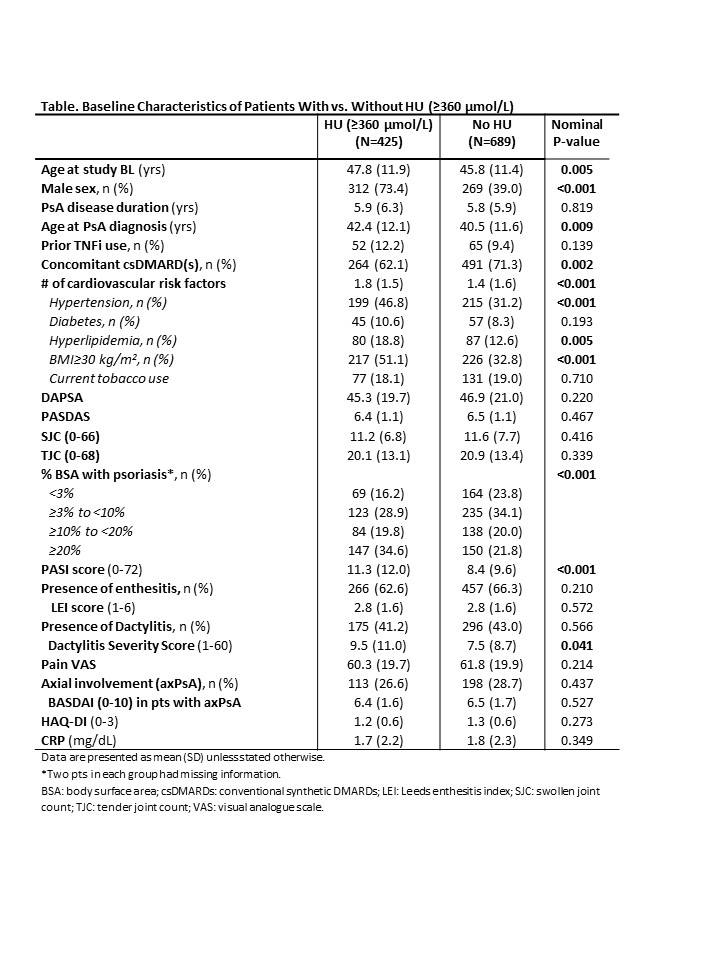
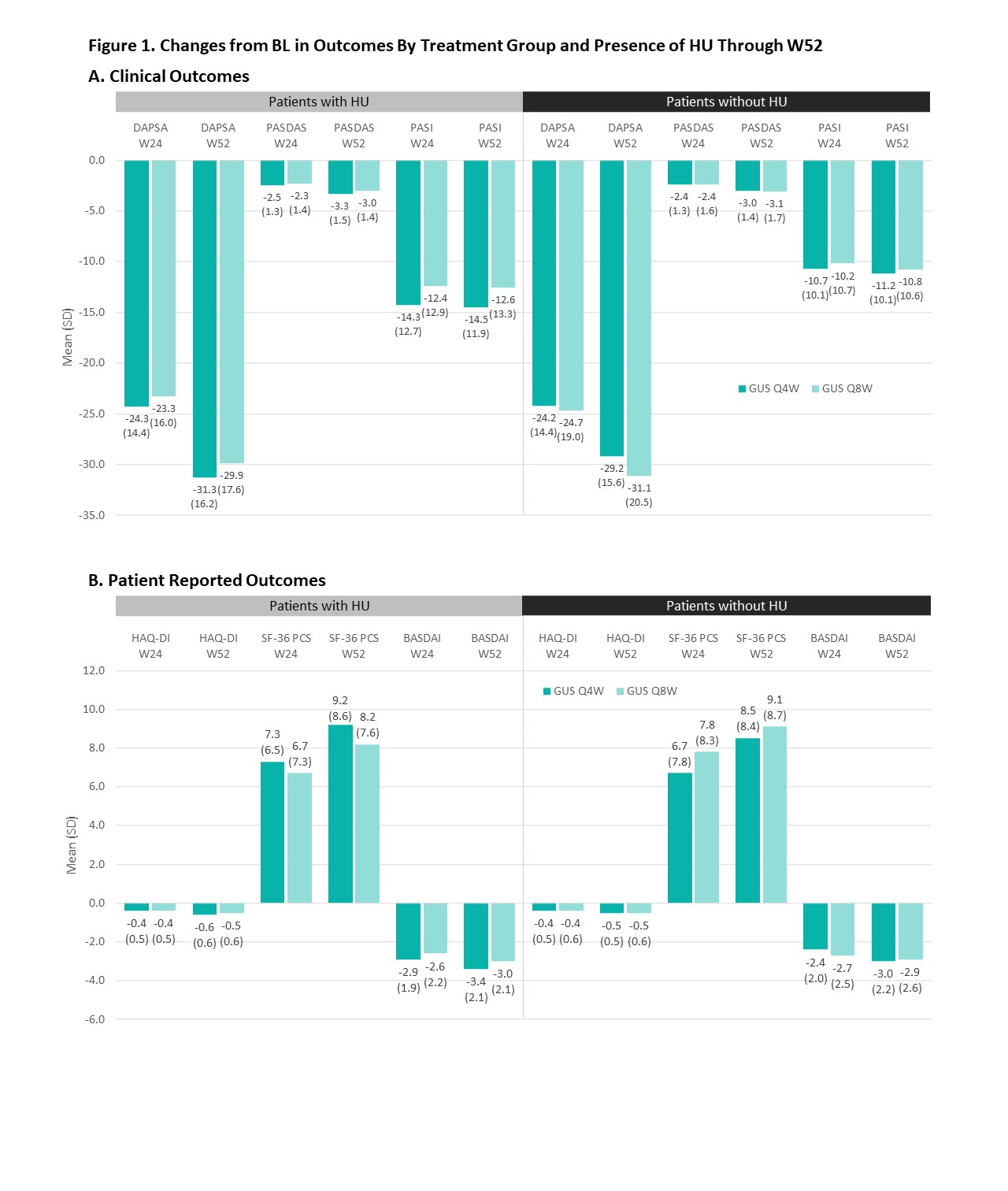
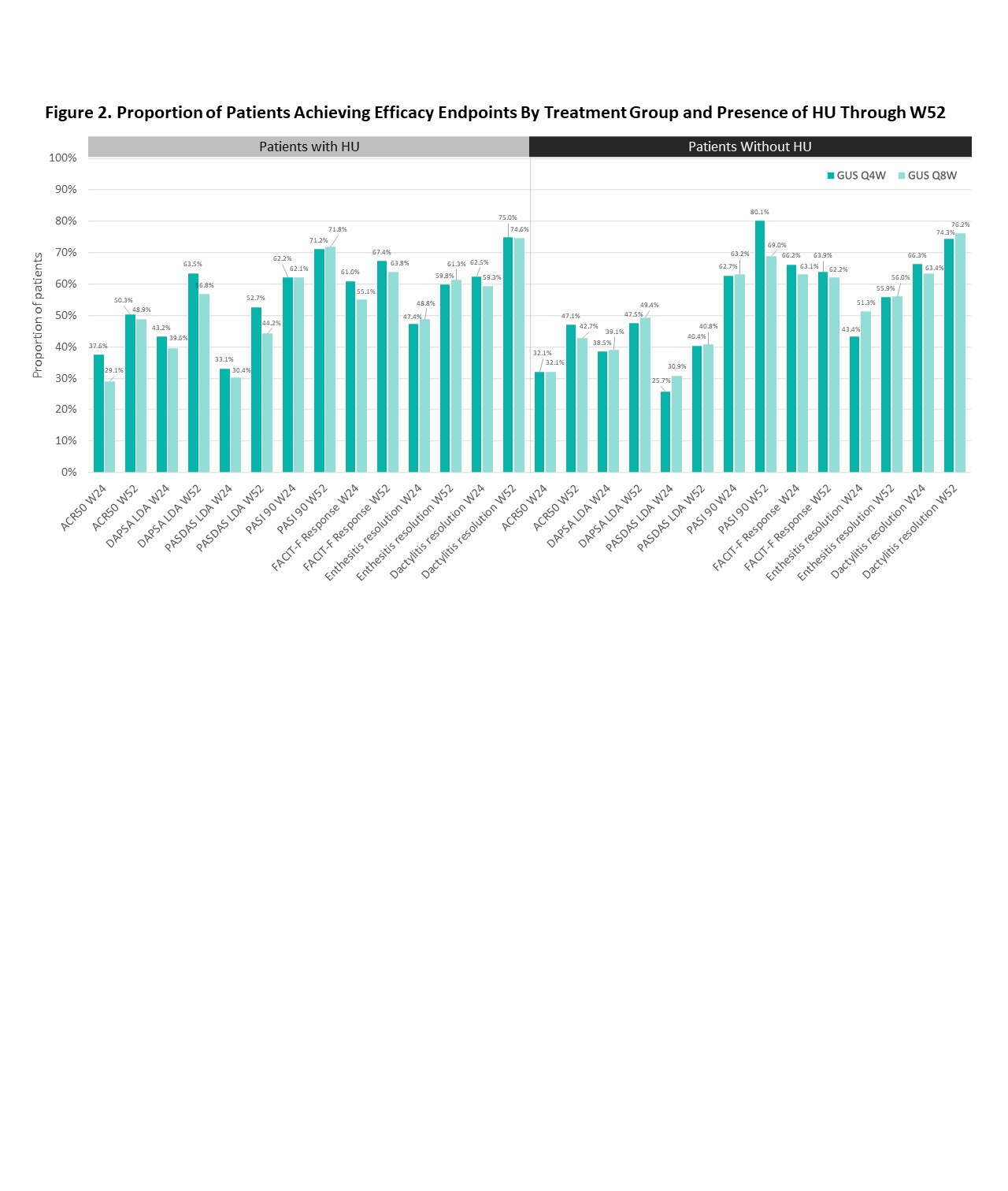
Results: Relative to no HU pts (n=689), those with HU (n=425) were older at study BL and PsA diagnosis, and more likely to be male and have hypertension, hyperlipidemia, BMI ≥30 kg/m2 and more severe PsO and dactylitis (in pts with dactylitis) (Table 1). The two cohorts had generally comparable severity of joint disease, enthesitis and axial symptoms. In multivariate analysis (not shown), male sex, hypertension, high BMI, and PsO severity were associated with HU. Clinically meaningful and comparable improvements in all studied continuous outcomes were observed at W24 across HU cohorts and GUS regimens (Figure 1); further improvements were generally seen through W52 regardless of HU. Endpoint achievement was also comparable between HU cohorts and GUS regimens (Figure 2).
Conclusion: In this pooled analysis of mainly bio-naïve pts with active PsA, HU pts were more likely to be male and have high BMI, hypertension, and more severe PsO and dactylitis. The observed differential clinical profile and comorbidities, confirmed in previous studies3, underscore the value of assessing serum urate levels in PsA pts. GUS was associated with clinically meaningful improvements across key PsA domains, with further enhancements generally seen through W52, irrespective of HU presence or GUS regimen.
References
1. Tripolino C. Front Med. 2021;8:737573
2. AlJohani R. J Rheum. 2018;45:213
3. Felten R. Ann Rheum Dis. 2022;81:855
To cite this abstract in AMA style:
FELTEN R, Widawski L, Spielman L, Gottenberg J, Duret P, Rampakakis E, Sharaf M, Constantin C, campana v, Messer L. Guselkumab Efficacy in Active Psoriatic Arthritis Patients with or Without Hyperuricemia: Post-hoc Analysis of Two Phase 3, Randomized, Double-blind, Placebo-Controlled Studies [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/guselkumab-efficacy-in-active-psoriatic-arthritis-patients-with-or-without-hyperuricemia-post-hoc-analysis-of-two-phase-3-randomized-double-blind-placebo-controlled-studies/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/guselkumab-efficacy-in-active-psoriatic-arthritis-patients-with-or-without-hyperuricemia-post-hoc-analysis-of-two-phase-3-randomized-double-blind-placebo-controlled-studies/