Session Information
Date: Tuesday, November 14, 2023
Title: (2227–2256) Spondyloarthritis Including Psoriatic Arthritis – Treatment: SpA Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Neutrophils have potential pathogenic roles in chronic inflammatory diseases (e.g., neutrophilic cutaneous microabscesses in psoriasis, and neutrophils in the synovial fluid/tissue in PsA and early sacroiliac joint biopsies in ankylosing spondylitis [AS]).1 An amplification mechanism involving neutrophilic IL-23 production, as part of the T-cell-neutrophil axis, has been theorized to associate with rapid-onset action of IL-23 pathway inhibition seen in some pts.2In these post hoc analyses of the Phase 3 DISCOVER-1&2 studies, we explored relationships between blood neutrophils and clinical outcomes during treatment with the fully human IL-23p19-subunit inhibitor guselkumab (GUS).
Methods: Adults in DISCOVER-1&2 (90% bio-naive) with active PsA were randomized 1:1:1 to GUS 100 mg every 4weeks (Q4W); GUS 100 mg at W0, W4, Q8W; or placebo (PBO). GUS effect (vs PBO) on early (W4) neutrophil (assessed as absolute count and neutrophil-to-lymphocyte ratio [NLR]) reduction was assessed using mixed models adjusting for potential confounders. In GUS patients (pts), associations between early (W4) neutrophil reduction and W24 achievement of 20% improvement in Bath AS Disease Activity Index (BASDAI20), ACR50, PsA Disease Activity Score (PASDAS) low/very low disease activity (LDA/VLDA), and enthesitis or dactylitis resolution were assessed with multivariate logistic regression. Associations between BL neutrophils and early clinical improvements (1st timepoint assessed) in swollen/tender joint counts (SJC/TJC at W4); skin visual analogue scale (VAS at W8); Leeds enthesitis index (LEI at W4); dactylitis severity score (DSS at W4); and spinal pain, BASDAI, and ASDAS in pts with axial involvement (axPsA; all at W8) were assessed in GUS pts using multivariate mixed models.
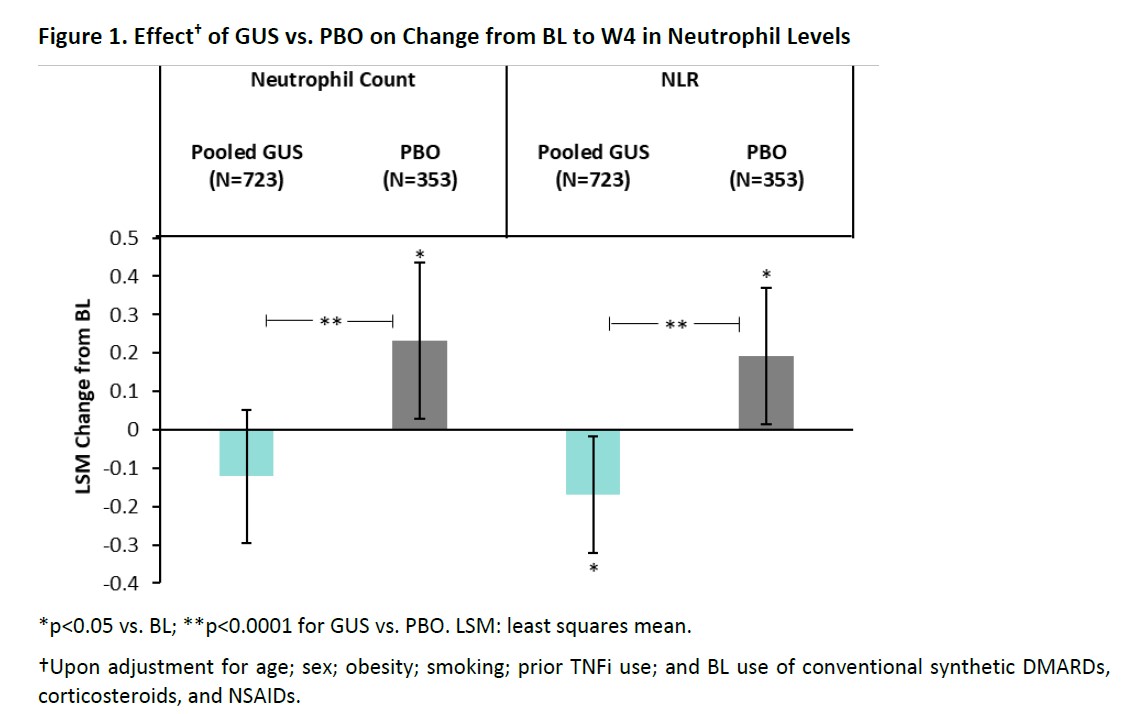
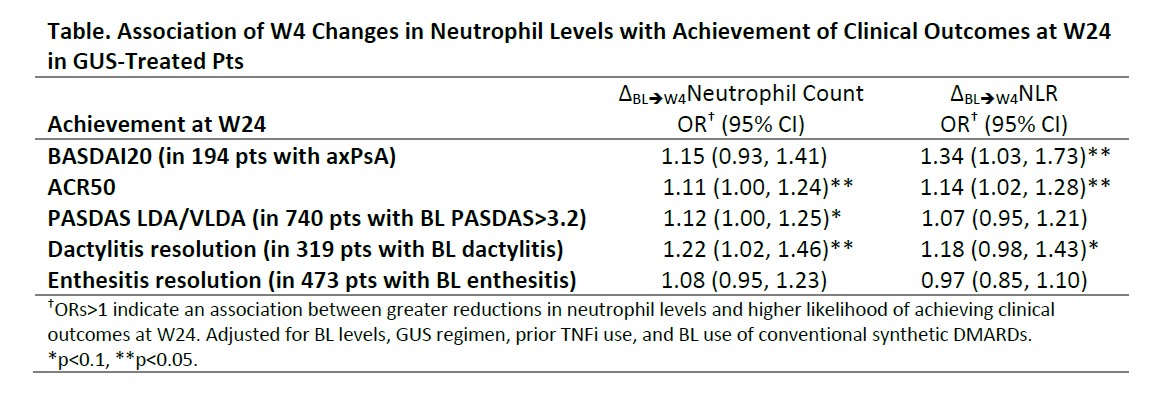
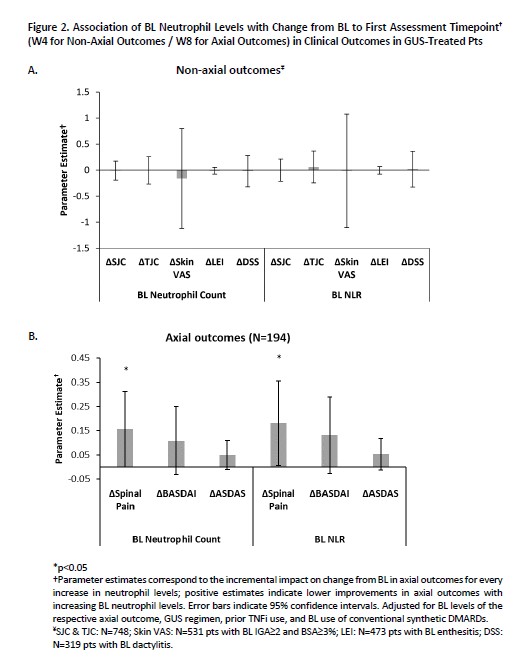
Results: GUS significantly reduced neutrophils (absolute count and NLR) vs PBO as early as W4 (Figure 1). Early (W4) reductions in neutrophils with GUS were associated with significantly higher odds of achieving BASDAI20; ACR50; PASDAS LDA/VLDA; and dactylitis, but not enthesitis, resolution at W24 (Table).However, no associations were observed between BL neutrophils in GUS-treated pts and early improvements in SJC, TJC, skin VAS, LEI, or DSS (Figure 2A); lower BL neutrophil levels, conversely, were associated with a significantly greater improvement in spinal pain, and numerically greater improvements in BASDAI and ASDAS, at W8 in axPsA pts (Figure 2B).
Conclusion: GUS rapidly (W4) reduced neutrophil levels, which in turn associated with greater likelihood of W24 achievement of important measures of disease control across PsA domains, e.g., ACR50, PASDAS LDA/VLDA, and dactylitis. The association between lower BL neutrophil levels and greater W8 improvement in spinal pain, but not with peripheral PsA domains, with GUS may signal a potential role of neutrophil reduction in mediating rapid improvements in axial symptoms and suggests a more impactful role for neutrophils in driving axial over peripheral symptoms. Such relationships thus require further study.
References:
1. Gong Y. Arthritis Rheum.2012;64:1399.
2. Macleod T. Lancet Rheumatol. 2023;5:47.
To cite this abstract in AMA style:
Macleod T, Baraliakos X, Rampakakis E, Chen W, Van Den Heuvel A, Seridi L, Shawi M, Lavie F, Merola J, Rahman P, McGonagle D. Neutrophil Levels Associate with Early Improvement in Spinal Pain and Week 24 Multi-Domain Disease Control During Guselkumab Treatment in Active Psoriatic Arthritis: Post Hoc Pooled Analyses of Two Phase 3 Randomized Controlled Trials [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/neutrophil-levels-associate-with-early-improvement-in-spinal-pain-and-week-24-multi-domain-disease-control-during-guselkumab-treatment-in-active-psoriatic-arthritis-post-hoc-pooled-analyses-of-two-ph/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/neutrophil-levels-associate-with-early-improvement-in-spinal-pain-and-week-24-multi-domain-disease-control-during-guselkumab-treatment-in-active-psoriatic-arthritis-post-hoc-pooled-analyses-of-two-ph/