Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: In patients with RA, high RF titers are considered a poor prognostic factor and are associated with higher disease activity, risk of radiographic progression, and decreased response to TNF inhibitors (TNFis).1–3 Recent data suggest that patients with RA and high RF titer may achieve and maintain greater clinical improvement with TNFis without a crystallizable fragment (Fc) compared to TNFis with an Fc.4 In this post hoc analysis of the EXXELERATE trial, we assessed efficacy outcomes of certolizumab pegol (CZP), a PEGylated Fc-free TNFi, versus adalimumab (ADA; Fc-containing TNFi) in patients with RA and high RF titers.
Methods: The phase 4 EXXELERATE trial (NCT01500278) compared the efficacy and safety of CZP to ADA in a head-to-head comparison; full study design and primary outcomes have been reported previously.5 Patients were randomized 1:1 to CZP 200 mg every 2 weeks (Q2W) plus methotrexate (MTX), or ADA 40 mg Q2W plus MTX. At Week (Wk) 12, patients were classified as responders or non-responders; non-responders were switched to the other TNFi with possible follow-up to Wk 104. Both Wk 12 responders and non-responders were included in this analysis. Here, we report drug plasma concentrations, mean disease activity score (DAS)28-CRP score, and proportion of patients achieving low disease activity (LDA; threshold: DAS28-CRP ≤2.7) to Wk 104. Results are stratified by RF titer quartile (≤Q3: ≤203 IU/mL; >Q3: >203 IU/mL; measured by Roche Tina-quant®) and reported as observed data.
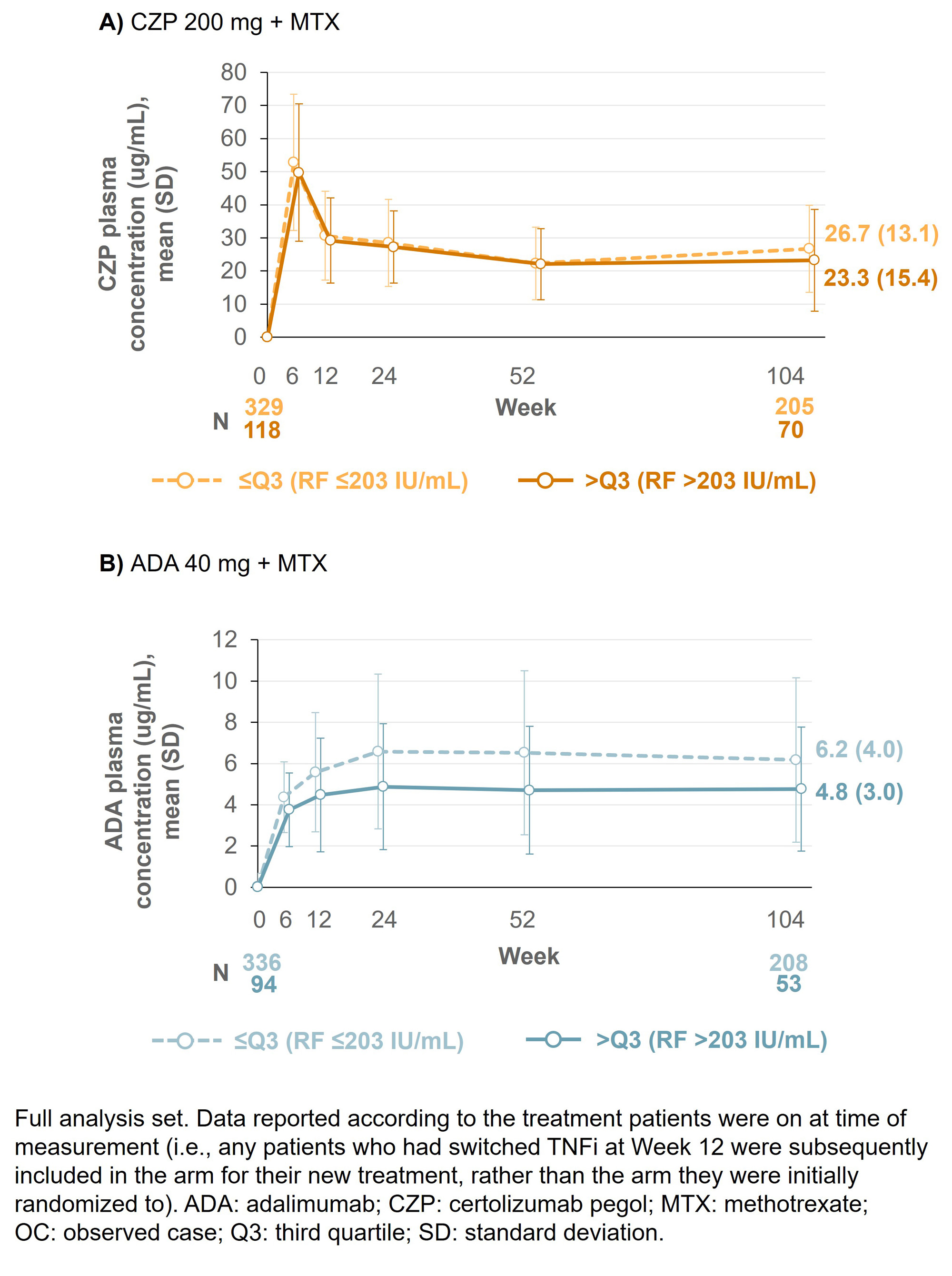
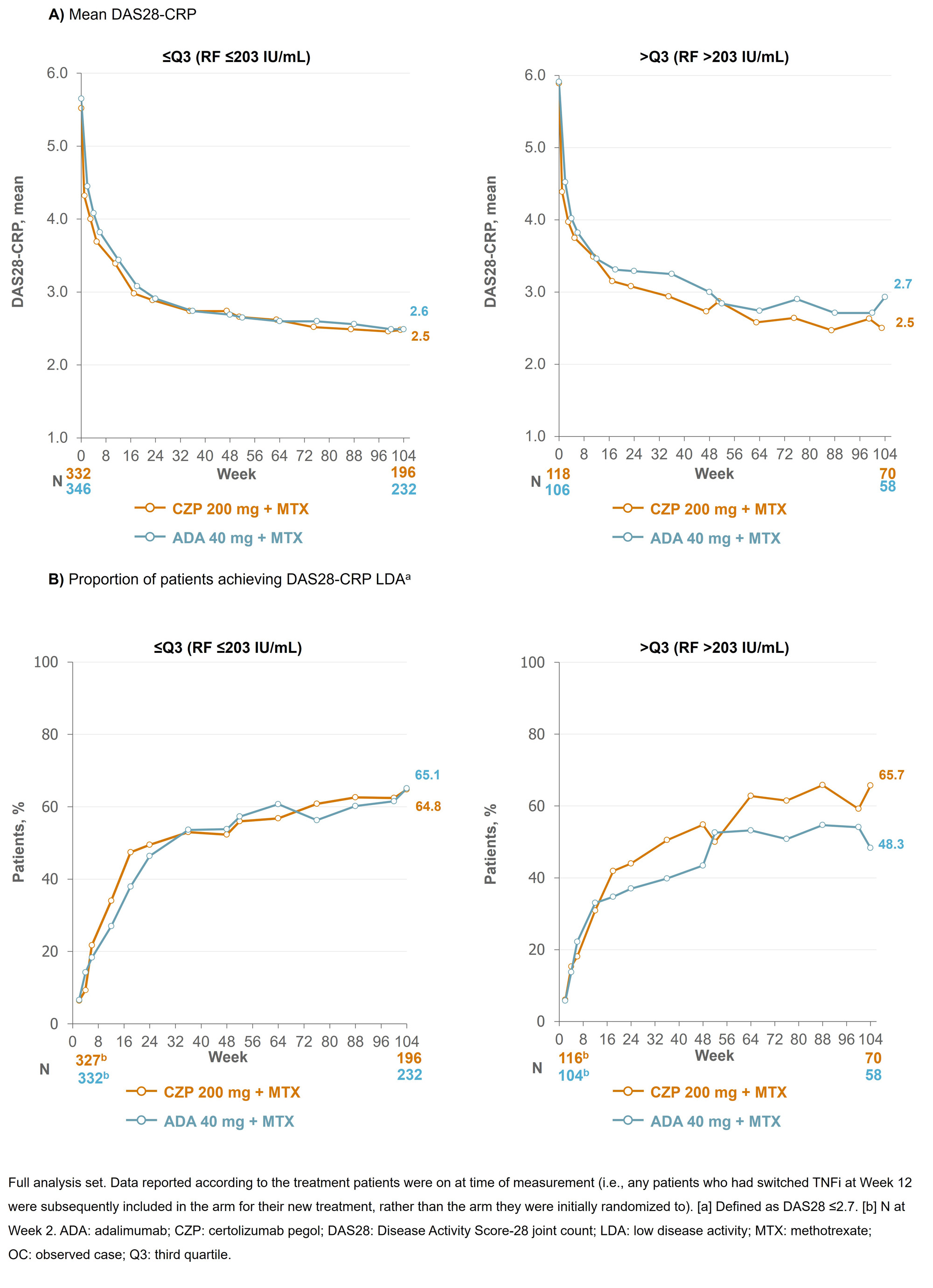
Results: Baseline (BL) data by RF quartile were available for 453 CZP-randomized patients (≤Q3: n=334; >Q3: n=119) and 454 ADA-randomized patients (≤Q3: n=347; >Q3 n=107). BL characteristics were similar between CZP- and ADA-randomized patients across the RF titer quartiles. At Wk 12, 66 CZP-treated patients switched to ADA and 59 ADA-treated patients switched to CZP. At Wk 104, mean ADA plasma concentrations were 22.9% lower in patients with RF >203 IU/mL vs those with RF ≤203 IU/mL; in CZP-treated patients, this difference was smaller (13.0%; Figure 1). For patients in RF ≤Q3, mean DAS28-CRP scores were similar between CZP- and ADA-treated patients through Wks 0–104 (mean [SD] DAS28 at Wk 104: 2.48 [1.18] CZP vs 2.49 [1.14] ADA). However, for patients in RF >Q3, mean DAS28-CRP scores were nominally lower in CZP- vs ADA-treated patients (Wk 104: 2.50 [1.18] CZP vs 2.93 [1.22] ADA; Figure 2). A similar pattern was observed for the proportion of patients achieving LDA at Wk 104 (≤Q3: 64.8% CZP vs 65.1% ADA; >Q3: 65.7% CZP vs 48.3% ADA).
Conclusion: CZP-treated patients with RA and high titer RF had similar drug concentrations and clinical responses to patients with RA and low titer RF, a pattern not observed in ADA-treated patients. These data, together with previous reports where CZP showed consistent efficacy irrespective of BL RF titer,6,7 suggest CZP may be a suitable therapy for patients with RA and high RF titer. References:1. Vastesaeger N. Rheumatology 2009;48:1114–21. 2. Cuchacovich M. Clin Rheumatol 2014;33:1707–14. 3. Takeuchi T. Arthritis Res Ther 2017;19:194. 4. Nakayama Y. Rheumatol Int 2022;42:1227–34. 5. Smolen J. Lancet 2016;388:2763–74. 6. Martínez-Feito A. Ann Rheum Dis 2022;81:594–5. 7. Tanaka Y. Int J Rheum Dis 2023;00:1–12.
To cite this abstract in AMA style:
Smolen J, Taylor P, Tanaka Y, Cara C, Lauwerys B, Xavier R, Curtis J, Mikuls T, Weinblatt M. Do High RF Titers Impact Response to TNF Inhibitors? Comparison of Certolizumab Pegol and Adalimumab in Patients with RA and High Titers of RF: A Post Hoc Analysis of a Phase 4 Trial [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/do-high-rf-titers-impact-response-to-tnf-inhibitors-comparison-of-certolizumab-pegol-and-adalimumab-in-patients-with-ra-and-high-titers-of-rf-a-post-hoc-analysis-of-a-phase-4-trial/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/do-high-rf-titers-impact-response-to-tnf-inhibitors-comparison-of-certolizumab-pegol-and-adalimumab-in-patients-with-ra-and-high-titers-of-rf-a-post-hoc-analysis-of-a-phase-4-trial/