Session Information
Date: Tuesday, November 14, 2023
Title: (1895–1912) Measures & Measurement of Healthcare Quality Poster II
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Systematic reviews of measurement properties of an outcome measurement instrument are fast becoming the evidence base for making decisions about the suitability of the instrument for a given application. Transparency in processes and decision-making at each step are important to allow consumers of the reviews to have a clear understanding of the results.At OMERACT (Outcome Measures in Rheumatology) we used an iterative process between methodologists and users for developing a summary of measurement properties table (SOMP) to use as a knowledge translation tool for communicating what was done, what was found, and what recommendations can be made from it.
Methods: Working with key stakeholders and end users, including patients, clinical trialists, clinicians and methodologists across several disease areas, the information that needed to be included in a SOMP was determined and initial designs laid out. Users provided feedback and revisions, which were integrated while ensuring the core elements were also being communicated.
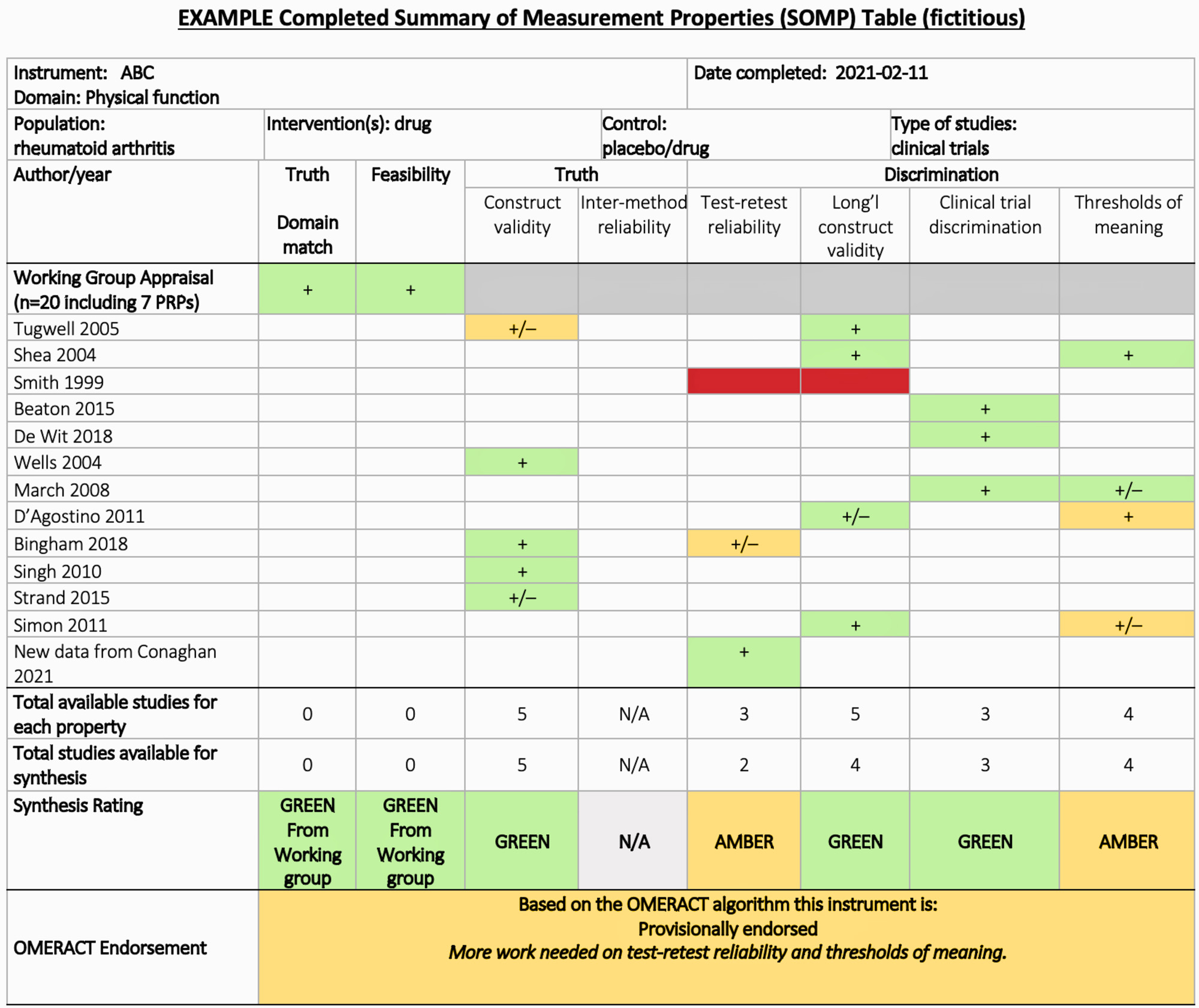
Results: Several features emerged for inclusion in the SOMP.We wanted the SOMP to be a standalone resource so the name/version of the tool, the target population (if restricted in the review) and the target concept the reviewers are trying to measure with this instrument were all included at the top of the table. Further, we designed the SOMP to be assembled as teams moved through the review steps so that it was clear the level of detail that was going into any statement about an instrument, and it was transparent exactly which studies/primary resources were included in the review and how they were reviewed.
The SOMP (Figure 1) uses symbols (colour for critical appraisal of methods, +/- for comparison of results to psychometric standards) and algorithms (for making conclusions based on the results in the synthesis steps).Should steps not be done in a given review (i.e., no critical appraisal in a descriptive review) the absence of colours in the boxes would demonstrate this for the users. Rows within the table include evidence from each study included in decision-making.
Conclusion: The SOMP was designed to capture the measurement properties evidence and decision-making processes for the OMERACT community to agree on whether an instrument is good enough to be included to represent a domain for a core outcome set.Its iterative development within a multidisciplinary consensus-based organization has helped us develop a tool we are finding useful in the transparent communication about methods and decision-making made in a given review.Although our key target is regulatory approval and core outcome set development, we have learned about the importance of being transparent in how decisions were made about an instrument, and how to share the body of evidence with others, leading to broader applications of the evidence base.
To cite this abstract in AMA style:
grosskleg s, maxwell l, Beaton D, Shea B, Tugwell P. Improving the Sharing of Information from Reviews of Measurement Properties: The OMERACT Summary of Measurement Properties (SOMP) Table [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/improving-the-sharing-of-information-from-reviews-of-measurement-properties-the-omeract-summary-of-measurement-properties-somp-table/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improving-the-sharing-of-information-from-reviews-of-measurement-properties-the-omeract-summary-of-measurement-properties-somp-table/