Session Information
Session Type: Abstract Submissions (ACR)
Abstract
Background/Purpose:
Tumor necrosis factor inhibitors (TNFi) are a mainstay in the treatment of rheumatoid arthritis (RA), as well as the management of spondyloarthritis (SpA) and inflammatory bowel disease (IBD). Unfortunately, a significant portion of patients taking these drugs require escalating doses to achieve response, while others lose response altogether. This may be due to the development of antibodies against TNFi.
Objectives:
To examine the immunogenicity of TNFi (adalimumab (ADAL), infliximab (IFX), etanercept (ETA), golimumab (GOL), and certolizumab pegol (CZP)) in RA, SpA, IBD, and to examine the potential effect of anti-drug antibodies (ADAB) on the loss of clinical response through a systematic literature review (SLR) and meta-analysis.
Methods:
A comprehensive literature search using 3 databases (Pubmed, Web of Science (WOS), and the Cochrane library) was conducted to identify studies examining the immunogenicity of TNFi in autoimmune diseases from 1966 and December 1, 2012. Studies eligible for inclusions required that they be in English, be randomized controlled trials, observational studies or case reports of more than 5 patients, and that patients were 18 years of age or older. Studies were excluded if they were strictly genetic with no clinical correlate, if the patients had concomitant cancer within 5 years of the study, or if the patients had a renal disease requiring dialysis. Double extraction was followed by a third extraction for disagreements. Random effect models were generated for the meta-analysis of the 48 studies to estimate the effects of ADAB on TNFi response.
Results:
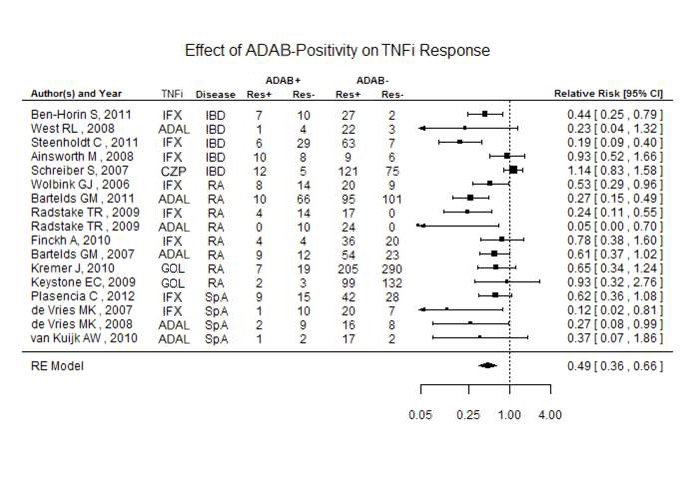
A total of 8861 patients from 48 studies matching the inclusion/exclusion criteria were examined. Trial durations were from 8 to 522 weeks. Overall, ADAB were detected in 18% [95% CI (0.14-0.23)] of the patients. 30% of those taking IFX developed ADAB [95% CI (0.23-0.37)], compared to 23% [95% CI (0.18-0.29)] for ADAL, 4% [95% CI (0.00-0.08)] for GOL, 4% [95% CI (0.08-0.16)] for ETA, and 6% [95% CI (0.02-0.11)] for CZP. The use of concomitant immunosuppressives (IS) (methotrexate (MTX), 6-mercaptopurine, azathioprine, and others) reduced ADAB formation in all patients by 31% [relative risk (RR) 0.69, 95% CI (0.59-0.81)]. Figure 1 shows that ADAB reduced clinical response by 51% overall, although most of the data derived from IFX (8 articles) and ADAL (6 articles). Under the random effect model, the summary effect for infliximab was RR 0.46 [95% CI (0.3-0.69)]; for ADAL the RR was 0.35 [95% CI (0.2-0.58)]; both estimates are statistically significant.
Conclusion:
All five TNFi are associated with anti-drug antibodies. Concomitant immunosuppresives reduced ADAB. ADAB are associated with a reduced clinical response.
Disclosure:
S. Thomas,
None;
N. Borazan,
None;
L. Duan,
None;
N. Barroso,
None;
S. Taroumian,
None;
B. Kretzmann,
None;
R. Bardales,
None;
D. Furst,
Abbott, Actelion, Amgen, BMS, Gilead, GSK, NIH, Novartis, Pfizer, Roche/Genentech, UCB,
2,
Abbott, Actelion, BMS, Biogen Idec, Centocor, CORRONA, Gilead, GSK, NIH, Novartis, Pfizer, Roche/Genentech, UCB,
5,
Abbott, Actelion, UCB,
8.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparative-immunogenicity-of-tumor-necrosis-factor-inhibitors-impact-on-clinical-efficacy-and-tolerability-in-the-management-of-autoimmune-diseases-a-systematic-review-and-meta-analysis/